The Project Gutenberg eBook of Neotropical Hylid Frogs, Genus Smilisca
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Neotropical Hylid Frogs, Genus Smilisca
Author: William Edward Duellman
Linda Trueb
Release date: October 22, 2011 [eBook #37823]
Language: English
Credits: Produced by Chris Curnow, Tom Cosmas, Joseph Cooper and
the Online Distributed Proofreading Team at
https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK NEOTROPICAL HYLID FROGS, GENUS SMILISCA ***

[Pg 281]
Museum of Natural History
Volume 17, No. 7, pp. 281-375, pls. 1-12, 17 figs.
July 14, 1966
Lawrence
1966
[Pg 282]
Editors: E. Raymond Hall, Chairman, Henry S. Fitch, Frank B. Cross
Volume 17, No. 7, pp. 281-375, pls. 1-12, 17 figs.
Published July 14, 1966
Lawrence, Kansas
ROBERT R. (BOB) SANDERS, STATE PRINTER
TOPEKA, KANSAS
1966
31-3430
[Pg 283]
| PAGE | |
| Introduction | 285 |
| Acknowledgments | 286 |
| Materials and Methods | 287 |
| Genus Smilisca Cope, 1865 | 287 |
| Key to Adults | 288 |
| Key to Tadpoles | 289 |
| Accounts of Species | 289 |
| Smilisca baudini (Duméril and Bibron) | 289 |
| Smilisca cyanosticta (Smith) | 303 |
| Smilisca phaeota (Cope) | 308 |
| Smilisca puma (Cope) | 314 |
| Smilisca sila New species | 318 |
| Smilisca sordida (Peters) | 323 |
| Analysis of Morphological Characters | 330 |
| Osteology | 330 |
| Descriptive Osteology of Smilisca baudini | 331 |
| Developmental Cranial Osteology of Smilisca baudini | 333 |
| Comparative Osteology | 336 |
| Musculature | 341 |
| Skin | 342 |
| Structure | 342 |
| Comparative Biochemistry of Proteins | 343 |
| External Morphological Characters | 343 |
| Size and Proportions | 343 |
| Shape of Snout | 344 |
| Hands and Feet | 344 |
| Ontogenetic Changes | 344 |
| Coloration | 344 |
| Metachrosis | 345 |
| Chromosomes | 345 |
| Natural History [Pg 284] | 345 |
| Breeding | 345 |
| Time of Breeding | 345 |
| Breeding Sites | 346 |
| Breeding Behavior | 346 |
| Breeding Call | 351 |
| Eggs | 356 |
| Tadpoles | 357 |
| General Structure | 357 |
| Comparison of Species | 357 |
| Growth and Development | 361 |
| Behavior | 365 |
| Phylogenetic Relationships | 366 |
| Interspecific Relationships | 366 |
| Evolutionary History | 369 |
| Summary and Conclusions | 371 |
| Literature Cited | 372 |
[Pg 285]
The family Hylidae, as currently recognized, is composed of
about 34 genera and more than 400 species. Most genera (30) and
about 350 species live in the American tropics. Hyla and 10 other
genera inhabit Central America; four of those 10 genera (Gastrotheca,
Hemiphractus, Phrynohyas, and Phyllomedusa) are widely
distributed in South America. The other six genera are either restricted
to Central America or have their greatest differentiation
there. Plectrohyla and Ptychohyla inhabit streams in the highlands
of southern Mexico and northern Central America; Diaglena and
Triprion are casque-headed inhabitants of arid regions in México
and northern Central America. Anotheca is a tree-hole breeder in
cloud forests in Middle America. The genus Smilisca is the most
widespread geographically and diverse ecologically of the Central
American genera.
The definition of genera in the family Hylidae is difficult owing
to the vast array of species, most of which are poorly known as
regards their osteology, colors in life, and modes of life history.
The genera Diaglena, Triprion, Tetraprion, Osteocephalus, Trachycephalus,
Aparasphenodon, Corythomantis, Hemiphractus, Pternohyla,
and Anotheca have been recognized as distinct from one
another and from the genus Hyla on the basis of various modifications
of dermal bones of the cranium. Phyllomedusa is recognized
on the basis of a vertical pupil and opposable thumb; Plectrohyla
is characterized by the presence of a bony prepollex and the absence
of a quadratojugal. Gastrotheca is distinguished from other
hylids by the presence of a pouch in the back of females. A pair
of lateral vocal sacs behind the angles of the jaws and the well-developed
dermal glands were used by Duellman (1956) to distinguish
Phrynohyas from Hyla. He (1963a) cited the ventrolateral
glands in breeding males as diagnostic of Ptychohyla. Some species
groups within the vaguely defined genus Hyla have equally distinctive
characters. The Hyla septentrionalis group is characterized
by a casque-head, not much different from that in the genus Osteocephalus
(Trueb, MS). Males in the Hyla maxima group have a
protruding bony prepollex like that characteristically found in
Plectrohyla.
Ontogenetic development, osteology, breeding call, behavior, and
ecology are important in the recognition of species. By utilizing
[Pg 286]
the combination of many morphological and biological factors, the
genus Smilisca can be defined reasonably well as a natural, phyletic
assemblage of species. Because the wealth of data pertaining to
the morphology and biology of Smilisca is lacking for most other
tree frogs in Middle America it is not possible at present to compare
Smilisca with related groups in more than a general way.
Smilisca is an excellent example of an Autochthonous Middle
American genus. As defined by Stuart (1950) the Autochthonous
Middle American fauna originated from "hanging relicts" left in
Central America by the ancestral fauna that moved into South
America and differentiated there at a time when South America
was isolated from North and Middle America. The genus Smilisca,
as we define it, consists of six species, all of which occur in Central
America. One species ranges northward to southern Texas, and
one extends southward on the Pacific lowlands of South America
to Ecuador. We consider the genus Smilisca to be composed of
rather generalized hylids. Consequently, an understanding of the
systematics and zoogeography of the genus can be expected to be
of aid in studying more specialized members of the family.
Acknowledgments
Examination of many of the specimens used in our study was possible only
because of the cooperation of the curators of many systematic collections. For
lending specimens or providing working space in their respective institutions
we are grateful to Doris M. Cochran, Alice G. C. Grandison, Jean Guibe, Robert
F. Inger, Günther Peters, Gerald Raun, William J. Riemer, Jay M. Savage,
Hobart M. Smith, Wilmer W. Tanner, Charles F. Walker, Ernest E. Williams,
and Richard G. Zweifel.
We are indebted to Charles J. Cole and Charles W. Myers for able assistance
in the field. The cooperation of Martin H. Moynihan at Barro Colorado
Island, Charles M. Keenan of Corozal, Canal Zone, and Robert Hunter of San
José, Costa Rica, is gratefully acknowledged. Jay M. Savage turned over to
us many Costa Rican specimens and aided greatly in our work in Costa Rica.
James A. Peters helped us locate sites of collections in Ecuador and Coleman
J. Goin provided a list of localities for the genus in Colombia.
We especially thank Charles J. Cole for contributing the information on the
chromosomes, and Robert R. Patterson for preparing osteological specimens.
We thank M. J. Fouquette, Jr., who read the section on breeding calls and
offered constructive criticism.
Permits for collecting were generously provided by Ing. Rodolfo Hernandez
Corzo in México, Sr. Jorge A. Ibarra in Guatemala, and Ing. Milton Lopez in
Costa Rica. This report was made possible by support from the National
Science Foundation (Grants G-9827 and GB-1441) and the cooperation of the
Museum of Natural History at the University of Kansas. Some of the field
studies were carried out in Panamá under the auspices of a grant from the
National Institutes of Health (NIH GM-12020) in cooperation with the Gorgas
Memorial Laboratory in Panamá.
[Pg 287]
Materials and Methods
In our study we examined 4151 preserved frogs, 93 skeletal preparations,
88 lots of tadpoles and young, and six lots of eggs. We have collected specimens
in the field of all of the species. Observations on behavior and life history
were begun by the senior author in México in 1956 and completed by us
in Central America in 1964 and 1965.
Osteological data were obtained from dried skeletons and
cleaned and
stained specimens of all species, plus serial sections of the skull of Smilisca
baudini. Developmental stages to which tadpoles are assigned are in accordance
with the table of development published by Gosner (1960). Breeding
calls were recorded in the field on tape using Magnemite and Uher portable
tape recorders. Audiospectrographs were made by means of a Vibralyzer (Kay
Electric Company). External morphological features were measured in the
manner described by Duellman (1956). In the accounts of the species we
have attempted to give a complete synonymy. At the end of each species
account the localities from which specimens were examined are listed alphabetically
within each state, province, or department, which in turn are listed
alphabetically within each country. The countries are arranged from north
to south. Abbreviations for museum specimens are listed below:
| AMNH—American Museum of Natural History BMNH—British Museum (Natural History) BYU—Brigham Young University CNHM—Chicago Natural History Museum KU—University of Kansas Museum of Natural History MCZ—Museum of Comparative Zoology MNHN—Museu National d'Histoire Naturelle, Paris UF—University of Florida Collections UIMNH—University of Illinois Museum of Natural History UMMZ—University of Michigan Museum of Zoology USC—University of Southern California USNM—United States National Museum TNHC—Texas Natural History Collection, University of Texas ZMB—Zoologisches Museum Berlin |
Genus Smilisca Cope, 1865
species Smilisca daulinia Cope, 1865 = Hyla baudini Duméril and Bibron,
1841]. Smith and Taylor, Bull. U. S. Natl. Mus., 194:75, June 17,
1948. Starrett, Copeia, 4:300, December 30, 1960. Goin, Ann. Carnegie
Museum, 36:15, July 14, 1961.
Definition.—Medium to large tree frogs having: (1) broad, well ossified
skull (consisting of a minimum amount of cartilage and/or secondarily ossified
cartilage), (2) no dermal co-ossification, (3) quadratojugal and internasal
septum present, (4) large ethmoid, (5) M. depressor mandibulae consisting
of two parts, one arising from dorsal fascia and other from posterior arm of
squamosal, (6) divided M. adductor mandibulae, (7) paired subgular vocal
sacs in males, (8) no dermal appendages, (9) pupil horizontally elliptical
(10) small amounts of amines and other active substances in skin, (11)
chromosome number of N = 12 and 2N = 24, (12) breeding call consisting of
poorly modulated, explosive notes, and (13) 2/3 tooth-rows in tadpoles.
Composition of genus.—As defined here the genus Smilisca contains six
recognizable species. An alphabetical list of the specific and subspecific names
[Pg 288]
that we consider to be applicable to species of Smilisca recognized herein is
given below.
| Names proposed | Valid names | |
| Hyla baudini Duméril and Bibron, 1841 | = S. baudini | |
| Hyla baudini dolomedes Barbour, 1923 | = S. phaeota | |
| Hyla beltrani Taylor, 1942 | = S. baudini | |
| Hyla gabbi Cope, 1876 | = S. sordida | |
| Hyla labialis Peters, 1863 | = S. phaeota | |
| Hyla manisorum Taylor, 1954 | = S. baudini | |
| Hyla muricolor Cope, 1862 | = S. baudini | |
| Hyla nigripes Cope, 1876 | = S. sordida | |
| Hyla pansosana Brocchi, 1877 | = S. baudini | |
| Hyla phaeota Cope, 1862 | = S. phaeota | |
| Hyla phaeota cyanosticta Smith, 1953 | = S. cyanosticta | |
| Hyla puma Cope, 1885 | = S. puma | |
| Hyla salvini Boulenger, 1882 | = S. sordida | |
| Hyla sordida Peters, 1863 | = S. sordida | |
| Hyla vanvlietii Baird, 1854 | = S. baudini | |
| Hyla vociferans Baird, 1859 | = S. baudini | |
| Hyla wellmanorum Taylor, 1952 | = S. puma |
Distribution of genus.—Most of lowlands of México and Central America,
in some places to elevations of nearly 2000 meters, southward from southern
Sonora and Río Grande Embayment of Texas, including such continental islands
as Isla Cozumel, México, and Isla Popa and Isla Cebaco, Panamá, to
northern South America, where known from Caribbean coastal regions and
valleys of Río Cauca and Río Magdalena in Colombia, and Pacific slopes of
Colombia and northern Ecuador.
Key to Adults
| 1. | Larger frogs ([M] to 76 mm., [F] to 90 mm.) having broad flat heads and a dark brown or black postorbital mark encompassing tympanum |
| 2 | |
| Smaller frogs ([M] to 45 mm., [F] to 64 mm.) having narrower heads and lacking a dark brown or black postorbital mark encompassing tympanum | |
| 4 | |
| 2. | Lips barred; flanks cream-colored with bold brown or black mottling in groin; posterior surfaces of thighs brown with cream-colored flecks |
| S. baudini, p. 289 | |
| Lips not barred; narrow white labial stripe present; flanks not cream-colored with bold brown or black mottling in groin; posterior surfaces of thighs variable | |
| 3 | |
| 3. | Flanks and anterior and posterior surfaces of thighs dark brown with large pale blue spots on flanks and small blue spots on thighs |
| S. cyanosticta, p. 303 | |
| Flanks cream-colored with fine black venation; posterior surfaces of thighs pale brown with or without darker flecks or small cream-colored spots | |
| S. phaeota, p. 308 | |
| 4. | Fingers having only vestige of web; diameter of tympanum two-thirds that of eye; dorsum pale yellowish tan with pair of broad dark brown stripes |
| S. puma, p. 314 | |
| Fingers about one-half webbed; diameter of tympanum about one-half that of eye; dorsum variously marked with spots or blotches | |
| 5 | |
| 5. | Snout short, truncate; vocal sacs in breeding males dark gray or brown; blue spots on flanks and posterior surfaces of thighs |
| S. sila, p. 318 | |
| Snout long, sloping, rounded; vocal sacs in breeding males white; cream-colored or pale blue flecks on flanks and posterior surfaces of thighs | |
| S. sordida, p. 323 | |
[Pg 289]
| 1. | Pond tadpoles; tail about half again as long as body; mouth anteroventral |
| 2 | |
| Stream tadpoles; tail about twice as long as body; mouth ventral | |
| 5 | |
| 2. | Labial papillae in two rows |
| 3 | |
| Labial papillae in one row | |
| 4 | |
| 3. | First upper tooth row strongly arched medially; third lower tooth row much shorter than other rows; dorsal fin deepest at about two-thirds length of tail; tail cream-colored with dense gray reticulations |
| S. puma, p. 314 | |
| First upper tooth row not arched medially; third lower tooth row nearly as long as others; dorsal fin deepest at about one-third length of tail; tail tan with brown flecks and blotches | |
| S. baudini, p. 289 | |
| 4. | Dorsal fin extending onto body |
| S. phaeota, p. 308 | |
| Dorsal fin not extending onto body | |
| S. cyanosticta, p. 303 | |
| 5. | Mouth completely bordered by two rows of papillae; inner margin of upper beak not forming continuous arch with lateral processes; red or reddish brown markings on tail |
| S. sordida, p. 323 | |
| Median part of upper lip bare; rest of mouth bordered by one row of papillae; inner margin of upper beak forming continuous arch with lateral processes; dark brown markings on tail | |
| S. sila, p. 318 | |
ACCOUNTS OF SPECIES
Smilisca baudini (Duméril and Bibron)
4798 from "Mexico;" Baudin collector]. Günther, Catalogue
Batrachia Salientia in British Museum, p. 105, 1858. Brocchi,
Mission scientifique au Mexique ..., pt. 3, sec. 2, Études sur les
batrachiens, p. 29, 1881. Boulenger, Catalogue Batrachia Salientia in
British Museum, p. 371, Feb. 1, 1882. Werner, Abhand. Zool.-Bot.
Gesell. Wien., 46:8, Sept. 30, 1896. Günther, Biologia Centrali-Americana:
Reptilia and Batrachia, p. 270, Sept. 1901. Werner, Abhand.
Konigl. Akad. Wiss. Munchen, 22:351, 1903. Cole and Barbour, Bull.
Mus. Comp. Zool., 50(5):154, Nov. 1906. Gadow, Through southern
México, p. 76, 1908. Ruthven, Zool. Jahr. 32(4):310, 1912. Decker,
Zoologica, 2:12, Oct., 1915. Stejneger and Barbour, A checklist of North
American amphibians and reptiles, p. 32, 1917. Noble, Bull. Amer. Mus.
Nat. Hist., 38(10):341, June 20, 1918. Nieden, Das Tierreich, Amphibia,
Anura I, p. 243, June, 1923. Gadow, Jorullo, p. 54, 1930. Dunn
and Emlen, Proc. Acad. Nat. Sci. Philadelphia, 84:24, March 22, 1932.
Kellogg, Bull. U. S. Natl. Mus., 160:160, March 31, 1932. Martin,
Aquarien Berlin, p. 92, 1933. Stuart, Occas. Papers Mus. Zool., Univ.
Michigan, 292:7, June 29, 1934; Misc. Publ. Mus. Zool. Univ. Michigan,
29:38, Oct. 1, 1935. Gaige, Carnegie Inst. Washington, 457:293, Feb.
5, 1936. Gaige, Hartweg, and Stuart, Occas. Papers Mus. Zool. Univ.
Michigan, 360:5, Nov. 20, 1937. Smith, Occas. Papers Mus. Zool. Univ.
Michigan, 388:2, 12, Oct. 31, 1938; Ann. Carnegie Mus., 27:312, March
14, 1939. Taylor, Copeia, 2:98, July 12, 1939. Hartweg and Oliver,
Misc. Publ. Mus. Zool. Univ. Michigan, 47:12, July 13, 1940. Schmidt
and Stuart, Zool. Ser. Field Mus. Nat. Hist., 24(21):238, August 30,
1941. Schmidt, Zool. Ser. Field Mus. Nat. Hist., 22(8):486, Dec. 30,
1941. Wright and Wright, Handbook of frogs and toads, Ed. 2, p. 134,
1942. Stuart, Occas. Papers Mus. Zool. Univ. Michigan, 471:15, May
17, 1943. Bogert and Oliver, Bull. Amer. Mus. Nat. Hist., 83(6):343,
March 30, 1945. Taylor and Smith, Proc. U. S. Natl. Mus., 95(3185): 590,
June 30, 1945. Smith, Ward's Nat. Sci. Bull., 1, p. 3, Sept., 1945. Schmidt
and Shannon, Fieldiana, Zool. Chicago Nat. Hist. Mus., 31(9):67, Feb.
[Pg 290]
20, 1947. Stuart, Misc. Publ. Mus. Zool. Univ. Michigan, 69:26, June
12, 1948. Wright and Wright, Handbook of frogs and toads, Ed. 3, p.
298, 1949. Stuart, Contr. Lab. Vert. Biol. Univ. Michigan, 45:22, May,
1950. Mertens, Senckenbergiana, 33:170, June 15, 1952; Abhand.
Senckenb. Naturf. Gesell., 487:28, Dec. 1, 1952. Schmidt, A checklist
of North American amphibians and reptiles, Ed. 6, p. 69, 1953. Stuart
Contr. Lab. Vert. Biol. Univ. Michigan, 68:46, Nov. 1954. Zweifel and
Norris, Amer. Midl. Nat., 54(1):232, July 1955. Martin, Amer. Nat.,
89:356, Dec. 1955. Duellman, Copeia, 1:49, Feb. 21, 1958. Goin,
Herpetologica, 14:119, July 23, 1958. Turner, Herpetologica, 14:192,
Dec. 1, 1958. Conant, A field guide to reptiles and amphibians, p. 284,
1958. Duellman, Univ. Kansas Publ., Mus. Nat. Hist., 13(2):59, Aug.
16, 1960; Univ. Kansas Publ., Mus. Nat. Hist., 15(1): 46, Dec. 20, 1961.
Porter, Herpetologica, 18:165, Oct. 17, 1962.
1854 [Holotype.—USNM 3256 from Brownsville, Cameron County,
Texas; S. Van Vliet collector]. Baird, United States and Mexican boundary
survey, 2:29, 1859. Smith and Taylor, Univ. Kansas Sci. Bull., 33:361,
March 20, 1950. Cochran, Bull. U. S. Natl. Mus., 220:60, 1961.
1859 [nomen nudum]. Diáz de León, Indice de los batracios que se
encuentran en la República Mexicana, p. 20, June 1904.
[Holotype.—USNM 25097 from Mirador, Veracruz, México; Charles
Sartorius collector]. Smith and Taylor, Univ. Kansas Sci. Bull., 33:349,
March 20, 1950. Cochran, Bull. U. S. Natl. Mus., 220:56, 1961.
1865 [Holotype.—"skeleton in private anatomical museum of Hyrtl, Professor
of Anatomy in the University of Vienna"]. Smith and Taylor, Univ.
Kansas Sci. Bull., 33:347, March 20, 1950.
23, pt. 2:205, 1871.
Sci. Philadelphia, 8, pt. 2:107, 1876; Proc. Amer. Philos. Soc., 18:267,
August 11, 1879. Yarrow, Bull. U. S. Nat. Mus., 24:176, July 1, 1882.
Cope, Bull. U. S. Nat. Mus., 32:13, 1887; Bull. U. S. Nat. Mus., 34:379,
April 9, 1889. Dickerson, The frog book, p. 151, July, 1906. Smith and
Taylor, Univ. Kansas Sci. Bull., 33:442, March 20, 1950; Taylor, U. Kan.
Sc. Bull., 34:802, Feb. 15, 1952; Univ. Kansas Sci. Bull., 35:794, July 1,
1952. Brattstrom, Herpetologica, 8(3):59, Nov. 1, 1952. Taylor, U. Kan.
Sci. Bull., 35:1592, Sept. 10, 1953. Peters, Occas. Papers Mus. Zool.
Univ. Michigan, 554:7, June 23, 1954. Duellman, Occas. Papers Mus.
Zool. Univ. Michigan, 560:8, Oct. 22, 1954. Chrapliwy and Fugler,
Herpetologica, 11:122, July 15, 1955. Smith and Van Gelder, Herpetologica,
11:145, July 15, 1955. Lewis and Johnson, Herpetologica, 11:178,
Nov. 30, 1955. Martin, Misc. Publ. Mus. Zool. Univ. Michigan, 101:53,
April 15, 1958. Stuart, Contr. Lab. Vert. Biol. Univ. Michigan, 75:17,
June, 1958. Minton and Smith, Herpetologica, 17:74, July 11, 1961. Nelson
and Hoyt, Herpetologica, 17:216, Oct. 9, 1961. Holman, Copeia, 2:256,
July 20, 1962. Stuart, Misc. Publ. Mus. Zool. Univ. Michigan, 122:41,
April 2, 1963. Maslin, Herpetologica, 19:124, July 3, 1963. Holman
and Birkenholz, Herpetologica, 19:144, July 3, 1963. Duellman, Univ.
Kansas Publ. Mus. Nat. Hist., 15(5):228, Oct. 4, 1963. Zweifel, Copeia,
1:206, March 26, 1964. Duellman and Klaas, Copeia, 2:313, June 30,
1964. Davis and Dixon, Herpetologica, 20:225, January 25, 1965. Neill,
Bull. Florida State Mus., 9:89, April 9, 1965.
6313 from Panzós, Alta Verapaz, Guatemala; M. Bocourt collector];
Mission scientifique au Mexique ..., pt. 3, sec. 2, Études
sur les batrachiens, p. 34, 1881.
[Pg 291]
amphibians and reptiles, Ed. 3, p. 34, 1933. Wright and Wright, Handbook
of frogs and toads, p. 110, 1933. Stejneger and Barbour, A checklist
of North American amphibians and reptiles, Ed. 4, p. 39, 1939; A
checklist of North American amphibians and reptiles, Ed. 5, p. 49, 1943.
Smith and Laufe, Trans. Kansas Acad. Sci., 48(3):328, Dec. 19, 1945.
Peters, Nat. Hist. Misc., 143:7, March 28, 1955.
[Holotype.—UIMNH 25046 (formerly EHT-HMS 29563) from Tapachula,
Chiapas, México; A. Magaña collector]. Smith and Taylor, Bull.
U. S. Natl. Mus. 194:87, June 17, 1948; Univ. Kansas Sci. Bull, 33:326,
March 20, 1950. Smith, Illinois Biol. Mono., 32:23, May, 1964.
Nov. 15, 1947. Smith and Taylor, Bull. U. S. Natl. Mus., 194:75, June
17, 1948; Univ. Kansas Sci. Bull., 33:347, March 20, 1950. Brown,
Baylor Univ. Studies, p. 68, 1950. Smith, Smith, and Werler, Texas
Jour. Sci., 4(2):254, June 30, 1952. Smith and Smith, Anales Inst. Biol.,
22(2):561, Aug. 7, 1952. Smith and Darling, Herpetologica, 8(3):82,
Nov. 1, 1952. Davis and Smith, Herpetologica, 8(4):148, Jan. 30, 1953.
Neill and Allen, Publ. Res. Div. Ross Allen's Reptile Inst., 2(1):26, Nov.
10, 1959. Maslin, Univ. Colorado Studies, Biol. Series, 9:4, Feb. 1963.
Holman, Herpetologica, 20:48, April 17, 1964.
[Holotype.—KU 34927 from Batán, Limón Province, Costa Rica; Edward
H. Taylor collector]. Duellman and Berg, Univ. Kansas Publ. Mus.
Nat. Hist, 15(4):193, Oct. 26, 1962.
Diagnosis.—Size large ([M] 76 mm., [F] 90 mm.); skull noticeably wider than
long, having small frontoparietal fontanelle (roofed with bone in large individuals);
postorbital processes long, pointed, curving along posterior border of
orbit; squamosal large, contacting maxillary; tarsal fold strong, full length of
tarsus; inner metatarsal tubercle large, high, elliptical; hind limbs relatively
short, tibia length less than 55 per cent snout-vent length; lips strongly barred
with brown and creamy tan; flanks pale cream with bold brown or black
reticulations in groin; posterior surfaces of thighs brown with cream-colored
flecks; dorsal surfaces of limbs marked with dark brown transverse bands.
(Foregoing combination of characters distinguishing S. baudini from any other
species in genus.)
Description and Variation.—Considerable variation in size, and in certain
proportions and structural characters was observed; variation in some characters
seems to show geographic trends, whereas variation in other characters
apparently is random. Noticeable variation is evident in coloration, but this
will be discussed later.
In order to analyze geographic variation in size and proportions, ten adult
males from each of 14 samples from various localities throughout the range of
the species were measured. Snout-vent length, length of the tibia in relation
to snout-vent length, and relative size of the tympanum to the eye are the only
measurements and proportions that vary noticeably (Table 1). The largest
specimens are from southern Sinaloa; individuals from the Atlantic lowlands
of Alta Verapaz in Guatemala, Honduras, and Costa Rica are somewhat smaller,
and most specimens from the Pacific lowlands of Central America are slightly
smaller than those from the Atlantic lowlands. The smallest males are from
the Atlantic lowlands of México, including Tamaulipas, Veracruz, the Yucatán
Peninsula, and British Honduras.
[Pg 292]
| Table 1.—Geographic Variation in Size and Proportions in Males of Smilisca baudini. (Means in Parentheses Below Observed Ranges; Data Based on 10 Specimens From Each Locality.) | |||
| Locality | Snout-vent length | Tibia length/ snout-vent | Tympanum/ eye |
| Southern Sinaloa | 62.3-75.9 | 43.2-46.7 | 84.2-94.4 |
| (68.6) | (44.9) | (87.8 | |
| Ocotito, Guerrero | 55.6-64.0 | 46.1-51.2 | 66.7-82.8 |
| (58.7) | (47.8) | (74.6) | |
| Pochutla, Oaxaca | 56.1-65.1 | 44.7-49.4 | 73.0-84.2 |
| (60.2) | (47.5) | (77.4) | |
| San Salvador, El Salvador | 57.0-68.0 | 42.1-46.1 | 74.6-83.3 |
| (62.1) | (44.9) | (77.6) | |
| Managua, Nicaragua | 52.9-63.6 | 45.6-49.4 | 73.7-89.7 |
| (57.3) | (47.5) | (79.4) | |
| Esparta, Costa Rica | 57.6-66.0 | 44.6-49.3 | 65.5-83.6 |
| (61.3) | (47.3) | (75.2) | |
| Ciudad Victoria, Tamaulipas | 50.6-56.9 | 44.5-48.7 | 67.2-84.3 |
| (53.7) | (46.6) | (73.9) | |
| Córdoba, Veracruz | 53.8-63.4 | 43.9-48.4 | 66.1-75.9 |
| (57.5) | (45.6) | (70.0) | |
| Isla del Carmen, Campeche | 47.3-56.6 | 44.7-48.9 | 61.5-72.6 |
| (50.9) | (47.6) | (65.7) | |
| Chichén-Itzá, Yucatán | 49.6-57.1 | 45.2-53.4 | 62.7-80.7 |
| (53.8) | (49.5) | (72.6) | |
| British Honduras | 49.0-59.6 | 47.5-50.7 | 67.9-76.8 |
| (54.9) | (49.1) | (72.2) | |
| Chinajá, Guatemala | 56.8-67.6 | 47.0-51.0 | 70.0-82. |
| (63.2) | (49.5) | (73.6 | |
| Atlantidad, Honduras | 52.5-65.1 | 49.8-53.6 | 56.1-76.5 |
| (57.6) | (51.5) | (67.0) | |
| Limón, Costa Rica | 57.7-71.3 | 50.4-52.3 | 63.9-73.0 |
| (62.4) | (51.2) | (68.5) | |
The ratio of the tibia to the snout-vent length varies from 42.1 to 53.6 in
the 14 samples analyzed. The average ratio in samples from the Pacific lowlands
varies from 44.9 in Sinaloa and El Salvador to 47.8 in Guerrero; on the
Gulf lowlands of México the average ratio varies from 45.6 in Veracruz to 47.6
on Isla del Carmen, Campeche. Specimens from the Yucatán Peninsula and
the Caribbean lowlands have relatively longer legs; the variation in average
ratios ranges from 49.1 in British Honduras to 51.2 in Costa Rica and 51.5 in
Honduras.
Specimens from southern Sinaloa are outstanding in the large size of the
[Pg 293]
tympanum; the tympanum/eye ratio varies from 84.2 to 94.4 (average 87.8).
In most other samples the variation in average ratios ranges from 72.2 to 79.3,
but specimens from Veracruz have an average ratio of 70.0; Campeche, 65.7;
Honduras, 67.0; and Limón, Costa Rica, 68.5.
No noticeable geographic trends in size and proportions are evident. Specimens
from southern Sinaloa are extreme in their large size, relatively short
tibia, and large tympani, but in size and relative length of the tibia the Sinaloan
frogs are approached by specimens from such far-removed localities as
San Salvador, El Salvador, and Chinajá, Guatemala. Frogs from the Caribbean
lowlands of Honduras and Costa Rica are relatively large and have relatively
long tibiae and small tympani.
The inner metatarsal tubercle is large and high and its shape varies. The
tubercle is most pronounced in specimens from northwestern México, Tamaulipas,
and the Pacific lowlands of Central America. Possibly the large tubercle
is associated with drier habitats, where perhaps the frogs use the tubercles for
digging.
The ground color of Smilisca baudini is pale green to brown dorsally and
white to creamy yellow ventrally. The dorsum is variously marked with dark
brown or dark olive-green spots or blotches (Pl. 6A). In most specimens a
dark interorbital bar extends across the head to the lateral edges of the eyelid;
usually this bar is connected medially to a large dorsal blotch. There is no
tendency for the markings on the dorsum to form transverse bands or longitudinal
bars. In specimens from the southern part of the range the dorsal
dark markings are often fragmented into small spots, especially posteriorly.
The limbs are marked by dark transverse bands, usually three on the forearm,
three on the thigh, and three or four on the shank. Transverse bands also
are present on the tarsi and proximal segments of the fingers and toes. The
webbing on the hands and feet is pale grayish brown. The loreal region and
upper lip are pale green or tan; the lip usually is boldly marked with broad
vertical dark brown bars, especially evident is the bar beneath the eye. A
dark brown or black mark extends from the tympanum to a point above the
insertion of the forearm; in some specimens this black mark is narrow or indistinct,
but in most individuals it is quite evident. The flanks are pale gray
to creamy white with brown or black mottling, which sometimes forms reticulations
enclosing white spots. The anterior surfaces of the thighs usually are
creamy white with brown mottling, whereas the posterior surfaces of the thighs
usually are brown with small cream-colored flecks. A distinct creamy white
anal stripe usually is present. Usually, there are no white stripes on the outer
edges of the tarsi and forearms. In breeding males the throat is gray.
Most variation in coloration does not seem to be correlated with geography.
The lips are strongly barred in specimens from throughout the range of the
species, except that in some specimens from southern Nicaragua and Costa
Rica the lips are pale and in some specimens the vertical bars are indistinct.
Six specimens from 7.3 kilometers southwest of Matatán, Sinaloa, are distinctively
marked. The dorsum is uniformly grayish green with the only dorsal
marks being on the tarsi; canthal and post-tympanic dark marks absent. A
broad white labial stripe is present and interrupted by a single vertical dark
mark below the eye. A white stripe is present on the outer edge of the foot.
The flanks and posterior surfaces of the thighs are creamy white, boldly marked
with black. Two specimens from Alta Verapaz, Guatemala (CNHM 21006
[Pg 294]
from Cobán and UMMZ 90908 from Finca Canihor), are distinctive in having
many narrow transverse bands on the limbs and fine reticulations on the flanks.
Two specimens from Limón Province, Costa Rica (KU 34927 from Batán and
36789 from Suretka), lack a dorsal pattern; instead these specimens are nearly
uniform brown above and have only a few small dark brown spots on the back
and lack transverse bands on the limbs. The post-tympanic dark marks and
dark mottling on the flanks are absent. Specimens lacking the usual dorsal
markings are known from scattered localities on the Caribbean lowlands from
Guatemala to Costa Rica.
The coloration in life is highly variable; much of the apparent variation is
due to metachrosis, for individuals of Smilisca baudini are capable of undergoing
drastic and rapid change in coloration. When active at night the frogs
usually are pale bright green with olive-green markings, olive-green with brown
markings, or pale brown with dark brown markings. The dark markings on
the back and dorsal surfaces of the limbs are narrowly outlined by black. The
pale area below the eye and just posterior to the broad suborbital dark bar is
creamy white, pale green, or ashy gray in life. The presence of this mark is
an excellent character by which to identify juveniles of the species. The flanks
are creamy yellow, or yellow with brown or black mottling. In most individuals
the belly is white, but in specimens from southern El Petén and northern
Alta Verapaz, Guatemala, the belly is yellow, especially posteriorly. The iris
varies from golden bronze to dull bronze with black reticulations, somewhat
darker ventrally.
Natural History.—Throughout most of its range Smilisca baudini occurs in
sub-humid habitats; consequently the activity is controlled by the seasonal nature
of the rainfall and usually extends from May or June through September.
Throughout México and Central America the species is known to call and
breed in June, July, and August. Several records indicate that the breeding
season in Central America is more lengthy. Gaige, Hartweg, and Stuart
(1937:4) noted gravid females collected at El Recreo, Nicaragua, in August
and September. Schmidt (1941:486) reported calling males in February in
British Honduras. Stuart (1958:17) stated that tadpoles were found in mid-February,
juveniles in February and March and half-grown individuals from
mid-March to mid-May at Tikal, El Petén, Guatemala. Stuart (1961:74)
reported juveniles from Tikal in July, and that individuals were active at night
when there had been light rain in the dry season in February and March in
El Petén, Guatemala. Smilisca baudini seeks daytime retreats in bromeliads,
elephant-ear plants (Xanthosoma), and beneath bark or in holes in trees. By
far the most utilized retreat in the dry season in parts of the range is beneath
the outer sheaths of banana plants. Large numbers of these frogs were found
in banana plants at Cuautlapan, Veracruz, in March, 1956, in March and December,
1959.
Large breeding congregations of this frog are often found at the time of
the first heavy rains in the wet season. Gadow (1908:76) estimated 45,000
frogs at one breeding site in Veracruz. In the vicinity of Tehuantepec, Oaxaca,
large numbers of individuals were found around rain pools and roadside ditches
in July, 1956, and July, 1958; large concentrations were found near Chinajá,
Guatemala, in June, 1960, and near Esparta, Costa Rica in July, 1961. Usually
males call from the ground at the edge of the water or not infrequently
sit in shallow water, but sometimes males call from bushes and low trees
[Pg 295]
around the water. Stuart (1935:38) recorded individuals calling and breeding
throughout the day at La Libertad, Guatemala. Smilisca baudini usually
is absent from breeding congregations of hylids; frequently S. baudini breeds
alone in small temporary pools separated from large ponds where numerous
other species are breeding. In Guerrero and Oaxaca, México, S. baudini breeds
in the same ponds with Rhinophrynus dorsalis, Bufo marmoreus, Engystomops
pustulosus, and Diaglena reticulata, and in the vicinity of Esparta, Costa Rica,
S. baudini breeds in ponds with Bufo coccifer, Hyla staufferi, and Phrynohyas
venulosa. In nearly all instances the breeding sites of S. baudini are shallow,
temporary pools.
The breeding call of Smilisca baudini consists of a series of short explosive
notes. Each note has a duration of 0.09 to 0.13 seconds; two to 15 notes make
up a call group. Individual call groups are spaced from about 15 seconds to
several minutes apart. The notes are moderately high-pitched and resemble
"wonk-wonk-wonk." Little vibration is discernible in the notes, which have
140 to 195 pulses per second and a dominant frequency of 2400 to 2725 cycles
per second (Pl. 10A).
The eggs are laid as a surface film on the water in temporary pools. The
only membrane enclosing the individual eggs is the vitelline membrane. In
ten eggs (KU 62154 from San Salvador, El Salvador) the average diameter
of the embryos in first cleavage is 1.3 mm. and of the vitelline membranes,
1.5 mm. Hatchling tadpoles have body lengths of 2.6 to 2.7 mm. and total
lengths of 5.1 to 5.4 mm. The body and caudal musculature is brown; the
fins are densely flecked with brown. The gills are long and filamentous.
Growth and development of tadpoles are summarized in Table 9.
A typical tadpole in stage 30 of development (KU 60018 from Chinajá,
Alta Verapaz, Guatemala) has a body length of 8.7 mm., a tail length of
13.6 mm., and a total length of 22.3 mm.; body slightly wider than deep;
snout rounded dorsally and laterally; eyes widely separated, directed dorsolaterally;
nostril about midway between eye and tip of snout; mouth anteroventral;
spiracle sinistral, located about midway on length of body and slightly
below midline; anal tube dextral; caudal musculature slender, slightly curved
upward distally; dorsal fin extending onto body, deepest at about one-third
length of tail; depth of dorsal fin slightly more than that of ventral fin at mid-length
of tail; dorsal part of body dark brown; pale crescent-shaped mark on
posterior part of body; ventral surfaces transparent with scattered brown pigment
ventrolaterally, especially below eye; caudal musculature pale tan with
a dark brown longitudinal streak on middle of anterior one-third of tail; dorsum
of anterior one-third of tail dark brown; brown flecks and blotches on
rest of caudal musculature, on all of dorsal fin, and on posterior two-thirds of
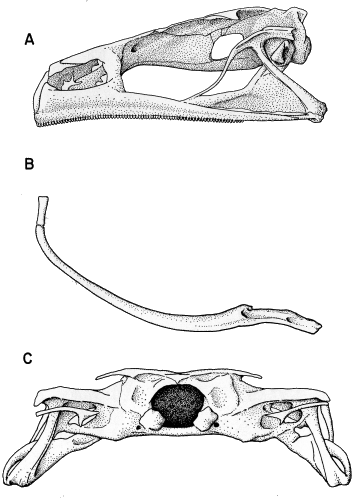
ventral fin; iris bronze in life (Fig. 11). Mouth small; median third of upper
lip bare; rest of mouth bordered by two rows of conical papillae; lateral fold
present; tooth rows 2/3; two upper rows about equal in length; second row
broadly interrupted medially, three lower rows complete, first and second equal
in length, slightly shorter than upper rows; third lower row shortest; first upper
row sharply curved anteriorly in midline; upper beak moderately deep, forming
a board arch with slender lateral processes; lower beak more slender,
broadly V-shaped; both beaks bearing blunt serrations (Fig. 15A).
In tadpoles having fully developed mouthparts the tooth-row formula of
2/3 is invariable, but the coloration is highly variable. The color and pattern
[Pg 296]
described above is about average. Some tadpoles are much darker, such as
those from 11 kilometers north of Vista Hermosa, Oaxaca, (KU 87639-44),
3.5 kilometers east of Yokdzonot, Yucatán (KU 71720), and 4 kilometers west-southwest
Puerto Juárez, Quintana Roo, México (KU 71721), whereas others,
notably from 17 kilometers northeast of Juchatengo, Oaxaca, México (KU
87645), are much paler and lack the dark markings on the caudal musculature.
The variation in intensity of pigmentation possibly can be correlated with environmental
conditions, especially the amount of light. In general, tadpoles
that were found in open, sunlit pools are pallid by comparison with those from
shaded forest pools. These subjective comparisons were made with preserved
specimens; detailed comparative data on living tadpoles are not available.
The relative length and depth of the tail are variable; in some individuals
the greatest depth of the tail is about at mid-length of the tail, whereas in
most specimens the tail is deepest at about one-third its length. The length
of the tail relative to the total length is usually 58 to 64 per cent in tadpoles in
stages 29 and 30 of development. In some individuals the tail is about 70
per cent of the total length. On the basis of the material examined, these
variations in proportions do not show geographical trends. Probably the proportions
are a reflection of crowding of the tadpoles in the pools where they
are developing or possibly due to water currents or other environmental factors.
Stuart (1948:26) described and illustrated the tadpole of Smilisca baudini
from Finca Chejel, Alta Verapaz, Guatemala. The description and figures
agree with ours, except that the first lower tooth row does not have a sharp
angle medially in Stuart's figure. He (1948:27) stated that color in tadpoles
from different localities probably varies with soil color and turbidity of water.
Maslin (1963:125) described and illustrated tadpoles of S. baudini from Pisté,
Yucatán, México. These specimens are heavily pigmented like specimens that
we have examined from the Yucatán Peninsula and from other places in the
range of the species. Maslin stated that the anal tube is median in the specimens
that he examined; we have not studied Maslin's specimens, but all tadpoles
of Smilisca that we have examined have a dextral anal tube.
Newly metamorphosed young have snout-vent lengths of 12.0 to 15.5 mm.
(average 13.4 in 23 specimens). The largest young are from La Libertad,
El Petén, Guatemala; these have snout-vent lengths of 14.0 to 15.5 mm. (average
14.5 in five specimens). Young from 11 kilometers north of Vista Hermosa,
Oaxaca, México, are the smallest and have snout-vent lengths of 12.0 to 12.5
mm. (average 12.3 in three specimens). Recently metamorphosed young
usually are dull olive green above and white below; brown transverse bands
are visible on the hind limbs. The labial markings characteristic of the adults
are represented only by a creamy white suborbital spot, which is a good diagnostic
mark for young of this species. In life the iris is pale gold.
Remarks: The considerable variation in color and the extensive geographic
distribution of Smilisca baudini have resulted in the proposal of eight specific
names for the frogs that we consider to represent one species. Duméril and
Bibron (1841:564) proposed the name Hyla baudini for a specimen (MNHN
4798) from México. Smith and Taylor (1950:347) restricted the type locality
to Córdoba, Veracruz, México, an area where the species occurs in abundance.
Baird (1854:61) named Hyla vanvlieti from Brownsville, Texas, and (1859:35)
labelled the figures of Hyla vanvlieti [= Hyla baudini] on plate 38 as Hyla
vociferans, a nomen nudum. Cope (1862:359) named Hyla muricolor from
Mirador, Veracruz, México, and (1865:194) used the name Smilisca daulinia
for a skeleton that he employed as the basis for the cranial characters diagnostic
of the genus Smilisca, as defined by him. Although we cannot be certain, Cope
apparently inadvertently used daulinia for baudini, just as he used daudinii for
baudini (1871:205). Brocchi (1877:125) named Hyla pansosana from Panzos,
Alta Verapaz, Guatemala.
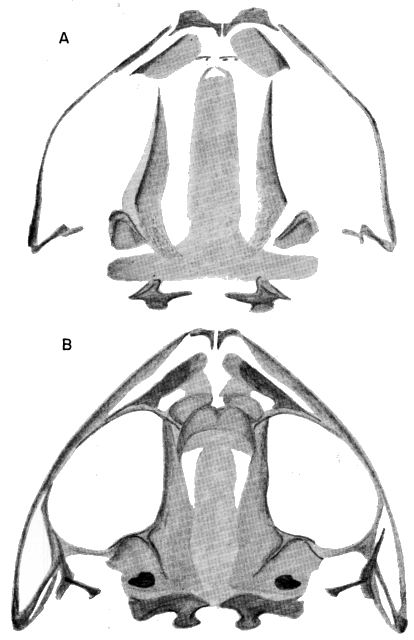
young (KU 60026), snout-vent length 12.6 mm. ×23; (B) young (KU 85438),
snout-vent length 32.1 mm. ×9.
(B) Ventral. ×4.5.
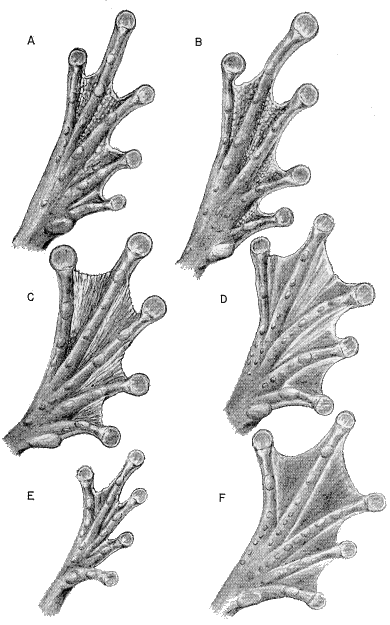
(B) Dorsal view of left mandible; (C) Posterior. ×4.5.
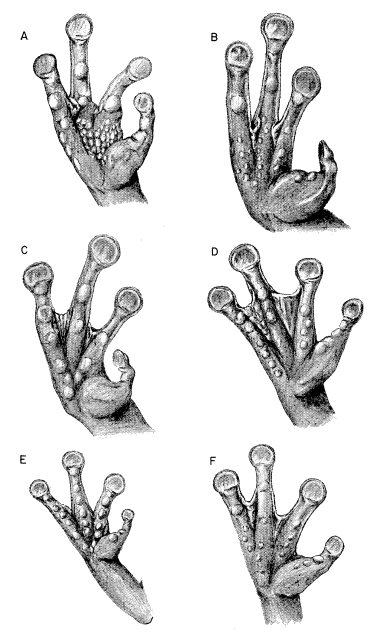
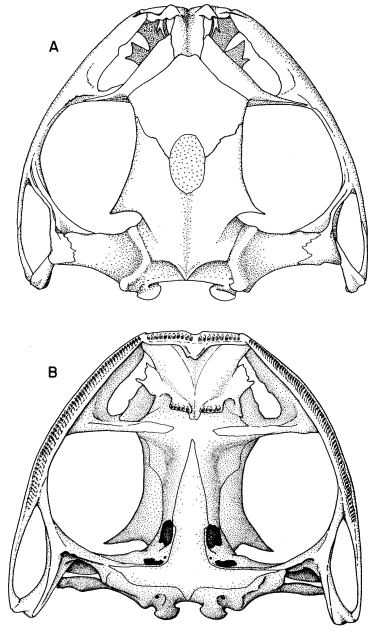
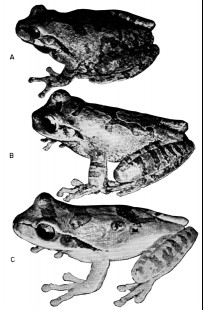
87177); (B) S. phaeota (KU 64276); (C) S. cyanosticta (KU
87199); (D) S. sordida (KU 91761); (E) S. puma (KU 91716),
and (F) S. sila (KU 77408). ×3.
S. phaeota (KU 64276); (C) S. cyanosticta (KU 87199); (D) S. sordida
(KU 91761); (E) S. puma (KU 91716), and (F) S. sila (KU 77408). ×3.
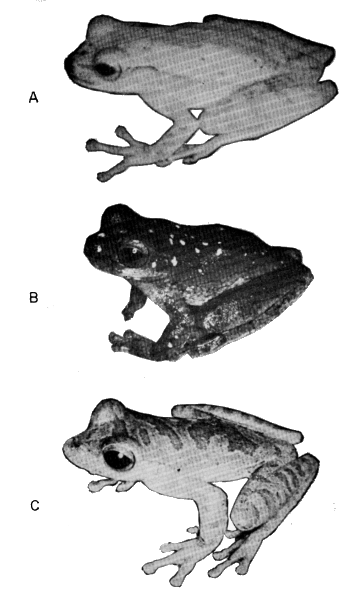
Xicotencatl, Tamaulipas, México; (B) S. cyanosticta (UMMZ 118163)
from Volcán San Martín, Veracruz, México; (C) S. phaeota (KU 64282)
from Barranca del Río Sarapiquí, Heredia Prov., Costa Rica. All approx.
nat. size.
W. Puerto Viejo, Heredia Prov., Costa Rica; (B) S.
sila (KU 77407) from Finca Palosanto, 6 km. WNW El
Volcán, Chiriquí, Panamá; (C) S. sordida (KU 64257)
from 20 km. WSW San Isidro el General, San José
Prov., Costa Rica. All approx. nat. size.

Prov., Costa Rica.

Rica.

Prov., Costa Rica.

Norte, Puntarenas Prov., Costa Rica.
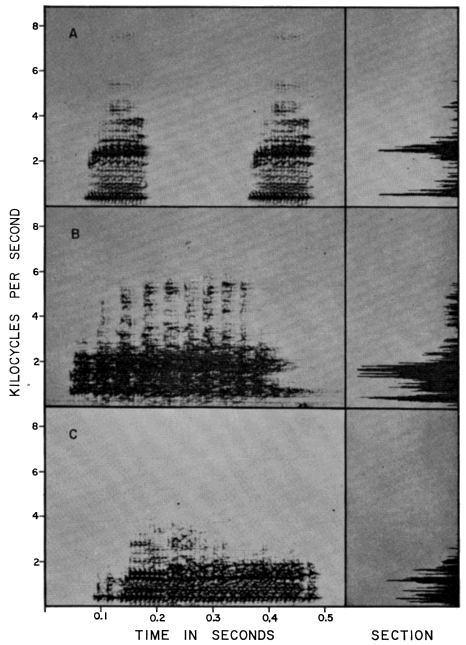
(KU Tape No. 74); (B) S. cyanosticta (KU Tape No. 373); (C) S. phaeota
(KU Tape No. 79).
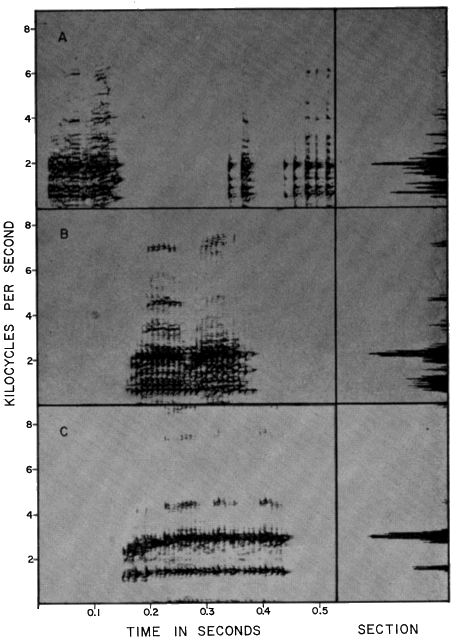
(KU Tape No. 382); (B) S. sila (KU Tape No. 385); (C) S. sordida
(KU Tape No. 398).
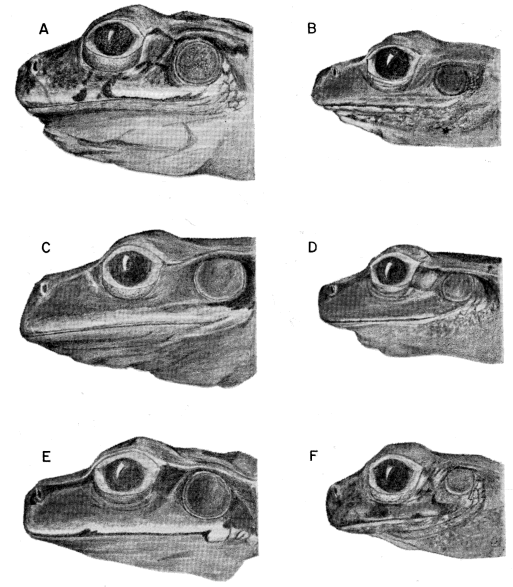
S. sordida (KU 91765); (C) S. phaeota (KU 64276); (D) S. puma (KU
91716); (E) S. cyanosticta (KU 87199); (F) S. sila (KU 77408). ×3.2.
[Pg 297]
Aside from the skeleton referred to as Smilisca daulinia by Cope (1865:194),
we have examined each of the types of the species synonymized with S.
baudini. All unquestionably are representatives of S. baudini.
Taylor (1942:306) named Hyla beltrani from Tapachula, Chiapas. This
specimen (UIMNH 25046) is a small female (snout-vent length, 44 mm.) of
S. baudini. Taylor (1954:630) named Hyla manisorum from Batán, Limón,
Costa Rica. The type (KU 34927) is a large female (snout-vent length,
75.3 mm.) S. baudini. In this specimen and a male from Suretka, Costa Rica,
the usual dorsal color pattern is absent, but the distinctive curved supraorbital
processes, together with other structural features, show that the two specimens
are S. baudini.
Hyla baudini dolomedes Barbour (1923:11), as shown by Dunn (1931a:413),
was based on a specimen of Smilisca phaeota from Río Esnápe, Darién,
Panamá.
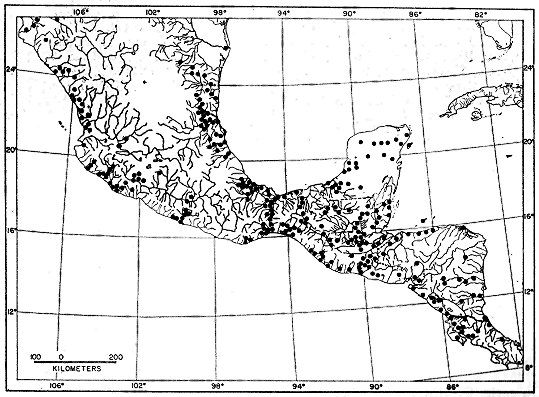
showing locality records for Smilisca baudini.
Distribution.—Smilisca baudini inhabits lowlands and foothills usually covered
by xerophytic vegetation or savannas, but in the southern part of its
range baudini inhabits tropical evergreen forest. The species ranges throughout
the Pacific and Atlantic lowlands of México from southern Sonora and the
Río Grande embayment of Texas southward to Costa Rica, where on the
Pacific lowlands the range terminates at the southern limits of the arid tropical
forest in the vicinity of Esparta; on the Caribbean lowlands the distribution
[Pg 298]
seems to be discontinuous southward to Suretka (Fig. 1). Most localities
where the species has been collected are at elevations of less than 1000 meters.
Three localities are notably higher; calling males were found at small temporary
ponds in pine-oak forest at Linda Vista, 2 kilometers northwest of
Pueblo Nuevo Solistahuacán, Chiapas, elevation 1675 meters, and 10 kilometers
northwest of Comitán, Chiapas, at an elevation of 1925 meters. Tadpoles and
metamorphosing young were obtained from a pond in arid scrub forest, 17
kilometers northeast of Juchatengo, Oaxaca, elevation 1600 meters. Stuart
(1954:46) recorded the species at elevations up to 1400 meters in the south-eastern
highlands of Guatemala.
Specimens examined.—3006, as follows: United States: Texas: Cameron
County, Brownsville, CNHM 5412-3, 6869, UMMZ 54036, USNM 3256.
Mexico: Campeche: Balchacaj, CNHM 102285, 102288, 102291, 102311,
UIMNH 30709-22, 30726; Champotón, UMMZ 73172 (2), 73176, 73180; 16
km. E Champotón, UMMZ 73181; 5 km. S Champotón, KU 71369-75; 9 km.
S Champotón, KU 71367-8; 10.5 km. S Champotón, KU 71365-6, 71722 (tadpoles),
71723 (yg.); 24 km. S Champotón, UMMZ 73177 (2); Chuina, KU 75101-3;
Ciudad del Carmen, UIMNH 30703-8; Dzibalchén, KU 75413-31; Encarnación,
CNHM 102282, 102289, 102294-5, 102300, 102306-8, 102312, 102314,
102316-7, 102319, 102322, UIMNH 30727-40, 30836-7; 1 km. W Escárcega,
KU 71391-6; 6 km. W Escárcega, KU 71397-403; 7.5 km. W Escárcega, KU
71376-89; 14 km. W Escárcega, KU 71390; 13 km. W, 1 km. N Escárcega,
KU 71404; 3 km. N Hopelchén, KU 75410-11; 2 km. NE Hopelchén, KU
75412; Matamoras, CNHM 36573; Pitál, UIMNH 30741; 1 km. SW Puerto
Real, Isla del Carmen, KU 71345-64; San José Carpizo, UMMZ 99879; Tres
Brazos, CNHM 102284, UIMNH 30723-5; Tuxpeña Camp, UMMZ 73239.
Chiapas: Acacoyagua, USNM 114487-92; 2 km. W Acacoyagua, USNM
114493-4; 5 km. E Arroyo Minas, UIMNH 9533-7; Berriozabal, UMMZ
119186 (7); Chiapa de Corzo, UMMZ 119185 (2); Cintalapa, UIMNH 50077;
Colonia Soconusco, USNM 114495-9; 5 km. W Colonia Soconusco, UMMZ
87885 (7); Comitán, UMMZ 94438; 10 km. NW Comitán, KU 57185; El
Suspiro, UMMZ 118819 (11); Escuintla, UMMZ 88271 (7), 88278, 88327,
109233; 6 km. NE Escuintla, UMMZ 87856 (26); 3 km. E Finca Juárez,
UIMNH 9538; Finca Prussia, UMMZ 95167; Honduras, UMMZ 94434-7;
La Grada, UMMZ 87862; 21 km. S La Trinitaria, UIMNH 9540-1; 14.4 km.
SW Las Cruces, KU 64239-44; Palenque, UIMNH 49286, USNM 114473-84;
2 km. NW Pueblo Nuevo Solistahuacán, KU 57182-4, UMMZ 119948 (8),
121514; 1.3 km. N Puerto Madero, KU 57186-9; 4 km. N Puerto Madero,
KU 57190-1; 8 km. N Puerto Madero, UMMZ 118379 (2); 12 km. N Puerto
Madero, KU 57192; 17.6 km. N Puerto Madero, UMMZ 118378; Rancho
Monserrata, UIMNH 9531-2, UMMZ 102266-7; Region Soconusco, UIMNH
33542-56; San Bartola, UIMNH 9519-30; San Gerónimo, UIMNH 30804;
San Juanito, USNM 114485-6; San Ricardo, CNHM 102406; Solosuchiapa,
KU 75432-3; Tapachula, CNHM 102208, 102219, 102239, 102405, UIMNH
25046, 30802-3; Tonolá, AMNH 531, CNHM 102232, 102416, UIMNH
30805-9, USNM 46760; Tuina, KU 41593 (skeleton); Tuxtla Gutierrez, CNHM
102231, 102248; 6 km. E Tuxtla Gutierrez, UIMNH 9539; 10 km. E Tuxtla
Gutierrez, UMMZ 119949.
Chihuahua: 2.4 km. SW Toquina, KU 47226-7; Riito, KU 47228.
Coahuila: mountain near Saltillo, UIMNH 30833-4.
Colima: No specific locality, CNHM 1632; Colima, AMNH 510-11; Hacienda
Albarradito, UMMZ 80029 (2); Hacienda del Colomo, AMNH 6208; Los
Mezcales, UMMZ 80028; Manzanillo, AMNH 6207, 6209; Paso del Río,
CNHM 102207, 102229-30, UIMNH 30819-21, UMMZ 110875 (3); Periquillo,
UMMZ 80025 (3), 80026 (14); 1.6 km. SW Pueblo Juárez, UMMZ 115564;
Queseria, CNHM 102204, 102216-7, 102224, UIMNH 30816-8, UMMZ 80023
(7), 80024 (7); Santiago, UMMZ 80027; 7.2 km. SW Tecolapa, UMMZ 115184.
[Pg 299]
Guerrero: Acahuizotla, UF 1338 (2), 1339-40, UMMZ 119182 (2), 119184;
3 km. S Acahuizotla, KU 87183-7; Acapulco, AMNH 55276, UMMZ 121879
(4), USNM 47909; 3 km. N Acapulco, UMMZ 110127; 8 km. NW Acapulco,
UF 11203 (7); 27 km. NE Acapulco, UIMNH 26597-610; Agua del Obispo,
CNHM 102214, 102290, 102293, 102310, 102413, KU 60413, 87180-2, UIMNH
30764-6; Atoyca, KU 87175-8; Buena Vista, CNHM 102279, 102304, 102313,
102315, UIMNH 30774; Caculutla, KU 87179; 20 km. S Chilpancingo, CNHM
102242, 102401, 102410-1, 102415; Colonia Buenas Aires, UMMZ 119189;
El Limoncito, CNHM 102292, 102303, 102321, 102414; El Treinte, CNHM
102212, 102221, 102237, 102240-1, UIMNH 30783-5, USNM 114508-10;
Laguna Coyuca, UMMZ 80960 (2); 3 km. N Mazatlán, UIMNH 30777-9; 9 km.
S Mazatlán, CNHM 102209, 102215, 102234, 102246, UIMNH 30781-2;
Mexcala, CNHM 102399, 102403, 102409, 106539-40, UIMNH 30775-6;
Ocotito, KU 60414-23; 5.4 km. N Ocotito, UMMZ 119181 (4); 1.6 km. N
Organos, UIMNH 30752-63; Palo Blanco, CNHM 102283, 102286, 102305,
102320, 102404, UIMNH 30767-70; Pie de la Cuesta, AMNH 55275, 59202-5;
Puerto Marquéz, AMNH 59200-1 (13); 5.6 km. S San Andreas de la Cruz,
KU 87173-4; San Vincente, KU 87172; Zaculapán, UMMZ 119183.
Hidalgo: Below Tianguistengo, CNHM 102318.
Jalisco: Atenqueque, KU 91435-6; 5 km. NE Autlán, UIMNH 30810; 5
km. E Barro de Navidad, UMMZ 110900; Charco Hondo, UMMZ 95247;
Puerto Vallarta, UIMNH 41346; between La Huerta and Tecomates, KU
91437; 3 km. SE La Resolana, KU 27619, 27620 (skeleton); 11 km. S, 1.6 km.
E Yahualica, KU 29039; Zapotilitic, CNHM 102238.
Michoacán: Aguililla, UMMZ 119179 (5); Apatzingán, CNHM 38766-90,
KU 69101 (skeleton); 7 km. E Apatzingán, UMMZ 112843; 11 km. E Apatzingán,
UMMZ 112841 (3); 27 km. S Apatzingán, KU 37621-3; 1.6 km. N
Arteaga, UMMZ 119180; Charapendo, UMMZ 112840; Coahuayana, UMMZ
104458; El Sabino, CNHM 102205-6, 102210-1, 102220, 102228, 102233,
UIMNH 30822-3; La Placita, UMMZ 104456; La Playa, UMMZ 105163; 30
km. E Nueva Italia, UMMZ 120255 (2); 4 km. S Nueva Italia, UMMZ 112842;
Ostula, UMMZ 104457 (4); Salitre de Estopilas, UMMZ 104459; San José de la
Montaña, UMMZ 104461 (2); 11 km. S Tumbiscatio, KU 37626; 12 km. S
Tzitzio, UMMZ 119178.
Morelos: 3.5 km. W Cuautlixco, KU 87188-90; 1 km. NE Puente de Ixtla,
KU 60393-4; 20 km. S Puente de Ixtla, CNHM 102400, UIMNH 30832;
Tequesquitengo, AMNH 52036-9.
Nayarit: 3 km. S Acaponeta, UMMZ 123030 (4); 56 km. S Esquinapa
(Sinaloa), KU 73909; Jesús María, AMNH 58239; San Blas, KU 28087, 37624,
62360-2, USNM 51408; 8.6 km. E San Blas, UMMZ 115185; Tepic, UIMNH
30812-5; 4 km. E Tuxpan, KU 67786; 11 km. SE Tuxpan, UIMNH 7329-31,
7335-59.
Nuevo León: Galeana, CNHM 34389; Salto de Cola de Caballo, CNHM
30628-31, 30632 (40), 30633-7, 34454-67.
Oaxaca: 11 km. S Candelaria, UIMNH 9515-8; Cerro San Pedro, 24 km.
SW Tehuantepec, UMMZ 82156; Chachalapa, KU 38199; 8 km. S Chiltepec,
KU 87191; 12 km. S Chivela, UMMZ 115182; Coyul, USNM 114512; Garza
Mora, UIMNH 40967-8; Juchatengo, KU 87193; 17 km. NE Juchatengo, KU
87645 (tadpoles), 87646 (young); Juchitán, USNM 70400; Lagartero, UIMNH
9514; Matías Romero, AMNH 52143-5; 25 km. N Matías Romero, KU 33822-8;
7 km. S Matías Romero, UIMNH 42703; Mirador, AMNH 6277, 13832-9,
13842-55; Mira León, 1.6 km. N Huatulco, UIMNH 9503-4; Mixtequillo,
AMNH 13924; Pochutla, KU 57167-81, UIMNH 9505-13; Quiengola, AMNH
51817, 52146; Río del Corte, UIMNH 48677; Río Mono Blanco, UIMNH
36831; Río Sarabia, 5 km. N Sarabia, UMMZ 115180 (4); 2.5 km. N Salina
Cruz, KU 57165-6; San Antonio, UIMNH 37286; 5 km. NNW San Gabriel
Mixtepec, KU 87192; San Pedro del Istmo, UIMNH 37197; Santo Domingo,
USNM 47120-2; 3.7 km. N Sarabia, UMMZ 115181 (3); Tapanatepec, KU
37793 (skeleton), 37794, UIMNH 9542, UMMZ 115183; between Tapanatepec
[Pg 300]
and Zanatepec, UIMNH 42704-25; Tecuane, UMMZ 82163 (3); Tehuantepec,
AMNH 52625, 52639, 53470, UMMZ 82157-8, 82159 (9), 82160 (4), 82161
(8), 82162 (12), 112844-5, 118703, USNM 10016, 30171-4, 30188; 4.5 km. W
Tehuantepec, KU 59801-12 (skeletons), 69102-3 (skeletons); 10 km. S Tehuantepec,
KU 57163-4; Temazcal, USC 8243 (3); 3 km. S Tolocita, KU 39666-9;
Tolosa, AMNH 53605; Tuxtepec, UMMZ 122098 (2); 2 km. S Valle Nacional,
KU 87194-5; 11 km. N Vista Hermosa, KU 87196, 87639-41 (tadpoles), 87642-3
(young), 87644 (tadpoles); Yetla, KU 87197.
Puebla: 16 km. SW Mecatepec (Veracruz), UIMNH 3657-8; San Diego,
AMNH 57714, USNM 114511; Vegas de Suchil, AMNH 57712; Villa Juárez,
UF 11205.
Quintana Roo: Cóba, CNHM 26937; Esmeralda, UMMZ 113551; 4 km.
NNE Felipe Carrillo Puerto, KU 71417-8; Pueblo Nuevo X-Can, KU 71405;
10 km. ENE Pueblo Nuevo X-Can, KU 71406; 4 km. WSW Puerto Juárez,
KU 71407-11, 71721 (tadpoles); 12 km. W Puerto Juárez, KU 71412-6; San
Miguel, Isla de Cozumel, UMMZ 78542 (6), 78543 (10), 78544 (2); 3.5 km. N
San Miguel, Isla de Cozumel, KU 71419-22; 10 km. E San Miguel, Isla de
Cozumel, UMMZ 78541; Telantunich, CNHM 26950.
San Luis Potosí: Ciudad Valles, AMNH 57776-81 (12), CNHM 37193,
102297, KU 23705; 21 km. N Ciudad Valles, UMMZ 118377; 6 km. E Ciudad
Valles, UF 3524; 24 km. E Ciudad Valles, UF 7340 (2); 5 km. S Ciudad Valles,
UIMNH 30751; 16 km. S Ciudad Valles, AMNH 52953; 30 km. S Ciudad
Valles, CNHM 102394, 102402, 102412, UIMNH 30749-50; 63 km. S Ciudad
Valles, UIMNH 19247-58; Pujal, UMMZ 99872 (2); Río Axtla, near Axtla,
AMNH 53211-5, 59516, KU 23706; Tamazunchale, AMNH 52675, CNHM
39621-2, 102226, 102281, UF 7615 (2), UIMNH 26596, UMMZ 99506 (9),
118701 (2), USNM 114468; 17 km. N Tamazunchale, UIMNH 3659; 2.4 km. S
Tamazunchale, AMNH 57743; 17 km. E Tamuin, UF 11202 (2); Xilitla,
UIMNH 19259-60.
Sinaloa: 8 km. N. Carrizalejo, KU 78133; 4 km. NE Concordia, KU 73914;
5 km. SW Concordia, KU 75438-9; 6 km. E Cosalá, KU 73910; Costa Rica,
16 km. S. Culiacán, UIMNH 34887-9; 51 km. SSE Culiacán, KU 37792; El
Dorado, KU 60392; 1.6 km. NE El Fuerte, CNHM 71468; Isla Palmito del
Verde, middle, KU 73916-7; 21 km. NNE Los Mochis, UIMNH 40536-7;
Matatán, KU 73913; 7.3 km. SW Matatán, KU 78464, 78466-70; Mazatlán,
AMNH 12562, UMMZ 115197 (3); 57 km. N Mazatlán, UIMNH 38364;
Plomosas, USNM 47439-40; Presidio, UIMNH 30811, USNM 14082; Rosario,
KU 73911-2; 5 km. E Rosario, UIMNH 7360-76; 8 km. SSE Rosario, KU 37625;
5 km. SW San Ignacio, KU 78465; 1.6 km. ENE San Lorenzo, KU 47917-24;
Teacapán, Isla Palmito del Verde, KU 73915; 9.6 km. NNW Teacapán, KU
91410; Villa Unión, KU 78471; 9 km. NE Villa Unión, KU 75434-7; 1 km.
W Villa Unión, AMNH 59284.
Sonora: Guiracoba, AMNH 51225-38 (25).
Tabasco: 4 km. NE Comalcalco, AMNH 60313; Teapa, UMMZ 119943;
5 km. N Teapa, UMMZ 119940, 119944, 122997 (2); 10 km. N Teapa, UMMZ
119187, 119188 (2); 13 km. N Teapa, UMMZ 119941 (2), 119945 (3), 120254
(2); 21 km. N Teapa, UMMZ 119942, 119947; 29 km. N Teapa, UMMZ 119946
(11); Tenosique, USNM 114505-7.
Tamaulipas: Acuña, UMMZ 99864; 5 km. S Acuña, UMMZ 101180; 13
km. N Antiguo Morelos, UIMNH 40532-5; 3 km. S Antiguo Morelos, UF
11204; 3 km. NE Chamal, UMMZ 102867; 1.6 km. E Chamal, UMMZ 110734;
Ciudad Mante, UMMZ 80957, 80958 (3), 106400 (3); 16 km. N Ciudad Victoria,
CNHM 102408; 34 km. NE Ciudad Victoria, KU 60395-411; 8.8 km. S Ciudad
Victoria, UIMNH 19261-3; 11 km. W Ciudad Victoria, UIMNH 30924; 16
km. W Ciudad Victoria, UIMNH 30825; 3 km. W El Carizo, UMMZ 111279;
Gómez Farías, UMMZ 110837-8; 8 km. NE Gómez Farías, UMMZ 102265,
102916 (4), 102917, 104110 (5), 105493, 110836 (2), 111274-7; 8 km. NW
Gómez Farías, UMMZ 101178 (7), 101179 (3), 101362-3, 101364 (2), 108799
(2), 110129, 111278, 111280; 8 km. W Gómez Farías, UMMZ 102859 (2); 16
km. W Gonzales, KU 37795-6; Jiménez, KU 60412; La Clementina, 6 km.
[Pg 301]
W Forlan, USNM 106244; Limón, UIMNH 30831; Llera, USNM 140137-40;
3 km. E Llera, UIMNH 16858; 21 km. S Llera, UIMNH 30828-9; 23 km.
S Llera, UIMNH 30830; 11 km. SW Ocampo, UMMZ 118956; 22 km. W, 5 km.
S Piedra, KU 37568-71; Rio Sabinas, UMMZ 97976; 5 km. W San Gerardo,
UMMZ 110733 (2); Santa Barbara, UMMZ 111272-3; Villagrán, CNHM 102280,
102287, 102299, 102309, UIMNH 30826-7; 1.7 km. W Xicotencatl, UMMZ
115179.
Veracruz: 1.6 km. NW Acayucan, UMMZ 115189; 28.5 km. SE Alvarado,
UMMZ 119933; 2.4 km. SSW Amatitlán, UMMZ 115195; Barranca Metlac,
UIMNH 38365; Boca del Río, UIMNH 26619-30, UMMZ 74954 (9); 16 km.
S Boca del Río, UIMNH 26631; between Boca del Río and Anton Lizardo,
UIMNH 42701; Canadá, CNHM 102397; Catemaco, UMMZ 118702 (4);
Ciudad Alemán, UMMZ 119608 (3); Córdoba, CNHM 38665-7, USNM 30410-3;
5.2 km. ESE Córdoba, KU 71423-35, 89924 (skeleton); 7 km. ESE Córdoba,
UMMZ 115176 (4); Cosamaloapan, UMMZ 115193 (2); Coyame, UIMNH
36853-6, 38366, UMMZ 111461 (3), 111462-3; 1 km. SE Coyame, UMMZ
121202 (3); Cuatotolapam, UMMZ 41625-39; Cuautlapan, CNHM 38664,
70591-600, 102218, 102398, KU 26300, 26302, 26309, 26312-3, 26315-6, 26321,
26336, 26339, 26347 (skeleton), 26354, 55614-21 (skeletons), UIMNH 11236-67,
11269-71, 11273, 26611-8, 30792-5, UMMZ 85466 (6), 115173 (25), 115175
(7), USNM 114433-57; Dos Ríos, CNHM 39623; 5 km. ENE El Jobo, KU
23843, 23845, 23847; 6.2 km. E Encero, UIMNH 30835; Escamilo, CNHM
102298, UIMNH 30788; 1 km. N Fortín, UF 11201; 4 km. SW Huatusco,
UMMZ 115177; 1 km. SW Huatusco, UMMZ 123119; 10 km. SE Hueyapan,
UMMZ 115190; 20 km. S Jesús Carranza, KU 23844, 23846, 27399; 38 km.
SE Jesús Carranza, KU 23417; Laguna Catemaco, UMMZ 119932 (62); 1.6
km. N La Laja, UIMNH 3651; La Oaxaqueña, AMNH 43930-1; 17 km. E
Martínez de la Torre, UIMNH 36630-2; 6.2 km. W Martínez de la Torre,
UIMNH 3652-4; Minatitlán, AMNH 52141-2; Mirador, USNM 25097-8,
115178; 6 km. S Monte Blanco, UF 11200 (4); 21 km. E Nanchital, UMMZ
123004; 2 km. S Naranja, UMMZ 115188 (3); 1.6 km. NE Novillero, UMMZ
115194 (2); 3 km. NE Novillero, UMMZ 115196; 5.2 km. NE Novillero,
UMMZ 115192 (4); 6 km. NE Novillero, UMMZ 115191; 5 km. N Nuevo
Colonia, UMMZ 105066; Orizaba, USNM 16563-6; 4 km. NE Orizaba, UMMZ
120251 (2); Panuco, UMMZ 118922; Paraje Nuevo, UMMZ 85465 (2), 85467
(35), 85468 (36); Paso del Macho, UIMNH 49281; Paso de Talaya, Jicaltepec,
USNM 32365, 84420; Pérez, CNHM 1686 (5); 20 km. N Piedras Negras, Río
Blanco, KU 23708; Plan del Río, KU 26310, 26333-5, 26345, 26354, UMMZ
102069, 102070 (5); Potrero, UIMNH 49282-5, UMMZ 88799, 88805, 88806
(2), USNM 32391-5; Potrero Viejo, CNHM 102296, KU 26301, 26304-5,
26307-8, 26311, 26317-20, 26323-25, 26326-8 (skeletons), 26329-31, 26332
(skeleton), 26337-8, 26340-4, 26346, 26348, 26351, 26353, 27400-12, UIMNH
30800, UMMZ 88800 (2), 88802 (15), 88803 (9), 88804, USNM 114458-67; 5
km. S Potrero Viejo, KU 26303, 26314, 26322; Puente Nacional, UIMNH
21783-8; 3 km. N Rinconada, UMMZ 122099 (5); Río de las Playas, USNM
118635-6; Río Seco, UMMZ 88801 (9); Rodriguez Clara, CNHM 102225; San
Andrés Tuxtla, CNHM 102213, 102222, 102227, 102247, UIMNH 30789-91;
10 km. NW San Andrés Tuxtla, UMMZ 119935; 13.4 km. NW San Andrés
Tuxtla, UMMZ 119939 (2); 19.8 km. NW San Andrés Tuxtla, UMMZ 119938;
27.2 km. NW San Andrés Tuxtla, UMMZ 119936 (6); 48 km. NW San Andrés
Tuxtla, UMMZ 119937; 4 km. W San Andrés Tuxtla, UMMZ 115187; 37.4
km. S San Andrés Tuxtla, UMMZ 119934 (12); 15 km. ESE San Juan de la
Punta, KU 23707; San Lorenzo, USNM 123508-12; 3 km. SW San Marcias
KU 23841; 1.5 km. S Santa Rosa, UIMNH 42702; 2 km. S Santiago Tuxtla,
UMMZ 121201 (4); Sauzel, UMMZ 121239; 14 km. E Suchil, UIMNH 46880;
15 km. S Tampico (Tamaulipas), UMMZ 103322 (4); 4 km. N Tapalapan,
UMMZ 115186 (2); Tecolutla, UIMNH 42677-700; 16 km. NW Tehuatlán,
UIMNH 3660-3; 5 km. S Tehuatlán, KU 23842; Teocelo, KU 26306; Tierra
Colorado, CNHM 102393, 102395-6, UIMNH 30789-91; Veracruz, AMNH
6301-4, 59398-402, UIMNH 30801, UMMZ 115174, 122060 (2); 24 km. W
Veracruz, CNHM 104570-2.
[Pg 302]
Yucatán: No specific locality, CNHM 548, 49067, USNM 32298; Chichén-Itzá,
CNHM 20636, 26938-49, 36559-62, UIMNH 30742-6, UMMZ 73173
(6), 73174 (14), 73175 (14), 73178-9, 76171, 83107 (2), 83108, 83109 (2), 83915
(30), USNM 72744; 9 km. E Chichén-Itzá, KU 71438-9; 12 km. E Chichén-Itzá,
KU 71440; Mérida, CNHM 40659-66, UIMNH 30747-8, UMMZ 73182;
6 km. S Mérida, KU 75194; 8.8 km. SE Ticul, UMMZ 114296; Valladolid,
CNHM 26934-6; Xcalah-op, CNHM 53906-14; 3.5 km. E Yokdzonot, KU
71441-3, 71720 (tadpoles).
British Honduras: Belize, CNHM 4153, 4384-5, 4387, UMMZ 75310,
USNM 26065; Bokowina, CNHM 49064-5; Cocquercote, UMMZ 75331 (2);
Cohune Ridge, UMMZ 80738 (15); Double Falls, CNHM 49066; El Cayo,
UMMZ 75311; 6 km. S El Cayo, MCZ 37856; Gallon Jug, MCZ 37848-55;
Manatee, CNHM 4264-7; Mountain Pine Ridge, MCZ 37857-8; San Augustin,
UMMZ 80739; San Pedro, Columbia, MCZ 37860-2; Valentin, UMMZ 80735
(4), 80736 (2), 80737 (2); 5 km. S Waha Loaf Creek, MCZ 37859.
Guatemala: Alta Verapaz: 5.1 km. NE Campur, KU 68464 (tadpoles),
67465 (young); 28.3 km. NE Campur, KU 64203-22, 68183-4 (skeletons);
Chamá, MCZ 15792-3, UMMZ 90895 (7), 90896 (5), 90897 (29), 90898 (12),
90899; Chinajá, KU 55939-41, 57193-8, 60018-20 (tadpoles), 60021 (eggs),
60022 (tadpoles); Cobán, CNHM 21006; Cubilquitz, UMMZ 90902 (10); Finca
Canihor, UMMZ 90908; Finca Chicoyou, KU 57246-8, 60026 (young), 64202,
68466-7 (tadpoles); Finca Los Alpes, KU 64197-201, 68463 (tadpoles); Finca
Los Pinales, UMMZ 90903 (2); Finca Tinajas, BYU 16031; Finca Volcán,
UMMZ 90905 (4), 90906-7; Panzos, MNHN 6313, UMMZ 90904; Samac,
UMMZ 90900; Samanzana, UMMZ 90901 (6).
Baja Verapaz: Chejel, UMMZ 90909 (7), 90910 (3); San Gerónimo, UMMZ
84076 (16).
Chiquimula: 1.6 km. SE Chiquimula, UMMZ 98112; Esquipulas, UMMZ
106793 (28).
El Petén: 20 km. NNW Chinajá (Alta Verapaz), KU 57199-240; Flores,
UMMZ 117985; La Libertad, KU 60024 (young), UMMZ 75313-20, 75323
(2), 75324 (7), 75325 (13), 75326 (2), 75327 (11), 75328 (12), 75329 (2); 3 km.
SE La Libertad, KU 57243-4; 13 km. S La Libertad, MCZ 21458 (2); Pacomon,
USNM 71334; Piedras Negras, USNM 114469-71; Poptún, UMMZ 120475;
Poza de la Jicotea, USNM 114672; Ramate-Yaxha trail, UMMZ 75321; Río de
la Pasión between Sayaxché and Subín, KU 57151; Río San Román, 16 km.
NNW Chinajá (Alta Verapaz), KU 55942-6; Sacluc, USNM 25131; Sayaxché,
KU 57144-5; Tikal, UMMZ 117983 (7), 117984 (5), 117993 (5), 120474 (5);
Toocog, KU 57241-2, 60023 (young), 60025 (young); Uaxactún, UMMZ
70401-3; Yaxha, UMMZ 75322; 19 km. E Yaxha, UMMZ 75330 (4).
El Quiché: Finca Tesoro, UMMZ 89166 (3), 90549 (tadpoles).
Escuintla: Río Guacalate, Masagua, USNM 125239; Tiquisate, UMMZ
98262 (7).
Guatemala: 16 km. NE Guatemala, KU 43545-53.
Huehuetenango: Finca San Rafael, 16 km. SE Barillas, CNHM 40912-6;
45 km. WNW Huehuetenango, KU 64223-4; Jacaltenango, UMMZ 120080
(6), 120081 (14), 120082 (13).
Izabál: 2 km. SW Puerto Matías de Gálvez, KU 60027-8 (tadpoles); Quiriguá,
CNHM 20587, UMMZ 70060.
Jalapa: Jalapa, UMMZ 98109, 106792 (11).
Jutiapa: Finca La Trinidad, UMMZ 107728 (10); Jutiapa, UMMZ 106789;
1.6 km. SE Mongoy, KU 43069; Santa Catarina Mita, UMMZ 106790.
Progreso: Finca Los Leones, UMMZ 106791.
Quetzaltenango: Coatepeque, AMNH 62204.
Retalhueleu: Casa Blanca, UMMZ 107725 (18); Champerico, UMMZ
107726 (3).
San Marcos: Talisman Bridge, USNM 128056-7.
[Pg 303]
Santa Rosa: Finca La Guardiana, UMMZ 107727 (6); Finca La Gloria,
UMMZ 107724 (6); 1.6 km. WSW El Molino, KU 43065-8.
El Salvador: La Libertad: 16 km. NW Santa Tecla, KU 43542-4.
Morazán: Divisadero, USNM 73284. San Salvador: San Salvador, CNHM
65087-99, KU 61955-88, 62138-9 (skeletons), 62154 (eggs), 62155-60 (tadpoles),
68462 (tadpoles), UMMZ 117586 (3), 118380 (3), USNM 140278.
Honduras: State unknown: Guaimas, UMMZ 58391. Atlantidad: Isla de
Roatán, CNHM 34551-4; La Ceiba, USNM 64985, 117589-91; Lancetilla,
MCZ 16207-11; Tela, MCZ 15774-5, 28080, UMMZ 58418, USNM 82173-4.
Choluteca: 1.5 km. NW Choluteca, KU 64228-32; 10 km. NW Choluteca, KU
64233; 10 km. E Choluteca, KU 64226-7; 12 km. E Choluteca, KU 64225; 5
km. S Choluteca, USC 2700 (2). Colón: Bambú, UF 320; Belfate, AMNH
45692-5; Patuca, USNM 20261. Comayagua: La Misión, 3.5 leagues N
Siguatepeque, MCZ 26424-5. Copán: Copán, UMMZ 83026 (2). Cortés:
Cofradía, AMNH 45345-6; Hacienda Santa Ana, CNHM 4724-31; Lago de
Yojoa, MCZ 26410-1; Río Lindo, AMNH 54972. El Paraiso: El Volcán, MCZ
26436. Francisco Morazán: Tegucigalapa, BYU 18301-4, 18837-41, MCZ
26395-7, USNM 60499. Gracias A Dios: Río Segovia, MCZ 24543. Santa
Barbara: Santa Barbara, USNM 128062-5.
Nicaragua: Chinandega: 4 km. N, 2 km. W Chichigalpa, KU 85385;
Chinandega, MCZ 2632; Río Tama, USNM 40022; San Antonio, KU 84944-9
(skeletons), 85386-403. Chontales: 1 km. NE Acoyapa, KU 64234. Estelí:
Finca Daraili, 5 km. N, 15 km. E Condega, KU 85404-8; Finca Venecia, 7
km. N, 16 km. E Condega, KU 85409. León: 1.6 km. ENE Poneloya, KU
43084-5. Managua: Managua, USNM 79989-90; 8 km. NW Managua, KU
43094-110; 20 km. NE Managua, KU 85412; 21 km. NE Managua, KU
85413-4; 5 km. SW Managua, KU 43086-93; 2 km. N Sabana Grande, KU
85411; 3 km. N Sabana Grande, KU 43070-8; 20 km. S, 0.5 km. W Tipitapa,
KU 85410. Matagalpa: Guasqualie, UMMZ 116493; Matagalpa, UMMZ
116492; 19 km. N Matagalpa, UMMZ 116494. Río San Juan: Greytown,
USNM 19585-6, 19767-8. Rivas: Javillo, UMMZ 123001; Moyogalpa, Isla
Ometepe, KU 85428-37, 87706 (tadpoles); Peñas Blancas, KU 85417; Río
Javillo, 3 km. N, 4 km. W Sapoá, KU 85418-20, 85438 (skeleton); 13.1 km.
SE Rivas, KU 85415; 14.8 km. SE Rivas, KU 85421-3; 11 km. S, 3 km. E
Rivas, KU 85416; 16 km. S Rivas, MCZ 29009-10; 7.7 km. NE San Juan del
Sur, KU 85426-7; 16.5 km. NE San Juan del Sur, KU 85424-5, 87705 (young);
5 km. SE San Pablo, KU 43079-83. Zelaya: Cooley, AMNM 7063-8, 8019-20,
8022, 8034-5; Cukra, AMNH 8016-7; Musahuas, Río Huaspuc, AMNH 58428-31;
11 km. NW Rama, Río Siquia, UMMZ 79708, 79709 (5), 79710 (2); Río
Escondido, USNM 19766, 20701; Río Siquia at Río Mico, UMMZ 79707 (10);
Sioux Plantation, AMNH 7058-61, 8023-33.
Costa Rica: Alajuela: Los Chiles, AMNH 54639; Orotina, MCZ 7960-1;
San Carlos, USNM 29991. Guanacaste: La Cruz, USC 8232 (3); 4.3 km. NE
La Cruz, UMMZ 123002; 18.4 km. S La Cruz, USC 8136; 23.5 km. S La Cruz,
USC 8094 (4); 3 km. W La Cruz, USC 8233 (4); 2 km. NE Las Cañas, KU
64235-7; Las Huecas, UMMZ 71212-3; Liberia, KU 36787, USC 8161; 11.5
km. N Liberia, USC 8149; 13 km. N Liberia, USC 8139; 22.4 km. N Liberia,
USC 8126; 8 km. NNW Liberia, KU 64238; 8.6 km. ESE Playa del Coco,
USC 8137; 21.8 km. ESE Playa del Coco, USC 8138; Río Piedra, 1.6 km. W
Bagaces, USC 7027; Río Bebedero, 5 km. S Bebedero, KU 64158; 5 km. NE
Tilarán, KU 36782-6. Heredia: 13 km. SW Puerto Viejo, KU 64142-6.
Limón: Batán, KU 34927; Guacimo, USC 621; Pandora, USC 505 (3); Suretka,
KU 36788-9; Tortugero, UF 7697, 10540-2. Puntarenas: Barranca, CNHM
35254-6; 15 km. WNW Barranca, KU 64155-7, UMMZ 118381; 18 km. WNW
Barranca, UMMZ 118382 (4); 4 km. WNW Esparta, KU 64159-96, 68178-82
(skeletons); 19 km. NW Esparta, KU 64147-54.
Smilisca cyanosticta (Smith), new combination
Taylor and Smith, Proc. U. S. Natl. Mus., 95(3185):589, June 30, 1945.
[Pg 304]
111147 from Piedras Negras, El Petén, Guatemala; Hobart
M. Smith collector]. Shannon and Werler, Trans. Kansas Acad. Sci.,
58:386, Sept. 24, 1955. Poglayen and Smith, Herpetologica, 14:11, April
25, 1958. Cochran, Bull. U. S. Natl. Mus., 220:57, 1961. Smith, Illinois
Biol. Mono., 32:25, May, 1964.
122:42, April 2, 1963. Duellman, Univ. Kansas Publ. Mus. Nat. Hist.,
15(5):229, Oct. 4, 1963.
Diagnosis.—Size moderately large ([M] 56.0 mm., [F] 70.0 mm.); skull as
long as wide; frontoparietal fontanelle large; narrow supraorbital flanges having
irregular margins anteriorly; large squamosal not in contact with maxillary;
tarsal fold moderately wide, full length of tarsus; inner metatarsal tubercle
moderately large, low, flat, elliptical; hind limbs relatively long; tibia usually
more than 52 per cent of snout-vent length; labial stripe silvery-white; lips
lacking vertical bars; loreal region pale green; pale bronze-colored stripe from
nostril along edge of eyelid to point above tympanum narrow, bordered below
by narrow dark brown stripe from nostril to eye, and broad dark brown
postorbital mark encompassing tympanum and terminating above insertion of
arm; flanks, dark brown with large pale blue spots; anterior and posterior
surfaces of thighs dark brown with small pale blue spots on thighs. (Foregoing
combination of characters distinguishing S. cyanosticta from any other species
in genus.)
Description and Variation.—The largest males are from Piedras Negras, El
Petén, Guatemala, and they average 52.5 mm. in snout-vent length whereas
males from Los Tuxtlas, Veracruz, average 50.6 mm. and those from northern
Oaxaca 50.3 mm. The smallest breeding male has a snout-vent length of 44.6
mm. The average ratio of tibia length to snout-vent length is 54.8 per cent
in males from Piedras Negras, and 56.4 and 56.3 per cent in males from Los
Tuxtlas and Oaxaca, respectively. The only other character showing noticeable
geographic variation is the size of the tympanum. The average ratio of the
diameter of the tympanum to the diameter of the eye is 76.3 per cent in males
from Piedras Negras, 71.8 from Oaxaca, and 66.9 from Los Tuxtlas.
The dorsal ground color of Smilisca cyanosticta is pale green to tan and
the venter is creamy white. The dorsum is variously marked with dark olive-green
or dark brown spots or blotches (Pl. 6B). An interorbital dark bar
usually is present. The most extensive dark area is a V-shaped mark in the
occipital region with the anterior branches not reaching the eyelids; this mark
is continuous, by means of a narrow mid-dorsal mark, with an inverted V-shaped
mark in the sacral region. In many specimens this dorsal marking is
interrupted, resulting in irregular spots. In some specimens the dorsum is
nearly uniform pale green or tan with a few small, dark spots. The hind limbs
are marked by dark transverse bands, usually three or four each on the thigh
and shank, and two or three on the tarsus. The webbing on the feet is brown.
The loreal region is pale green, bordered above by a narrow, dark brown
canthal stripe extending from the nostril to the orbit, which is bordered above
by a narrow, bronze-colored stripe extending from the nostril along the edge
of the eyelid to a point above the tympanum. The upper lip is white. A
broad dark brown mark extends posteriorly from the orbit and encompasses the
tympanum to a point above the insertion of the forelimb. The flanks are dark
brown with many pale blue, rounded spots, giving the impression of a pale
[Pg 305]
blue ground color with dark brown mottling enclosing spots. The anterior
and posterior surfaces of the thighs are dark brown with many small pale
blue spots. The inner surfaces of the shank and tarsus are colored like the
posterior surfaces of the thighs. Pale blue spots are usually present on the
proximal segments of the second and third toes. A distinct white stripe is
present on the outer edge of the tarsus and fifth toe; on the tarsus the white
stripe is bordered below by dark brown. A white stripe also is present on the
outer edge of the forearm and fourth finger. The anal region is dark brown,
bordered above by a narrow transverse white stripe. The throat in breeding
males is dark, grayish brown with white flecks.
No geographic variation in the dorsal coloration is evident. Specimens from
the eastern part of the range (Piedras Negras and Chinajá, Guatemala) have
bold, dark reticulations on the flanks enclosing large pale blue or pale green
spots, which fade to tan in preservative. Specimens from Oaxaca and Veracruz
characteristically have finer dark reticulations on the flanks enclosing smaller
blue spots; in many of these specimens the ventrolateral spots are smallest and
are white.
All living adults are easily recognized by the presence of pale, usually blue,
spots on the flanks and thighs. Individuals under cover by day have a tan
dorsum with dark brown markings. A hiding individual at Chinajá, Alta
Verapaz, Guatemala (KU 55936), had a pale tan dorsum when found; later
the dorsal color changed to chocolate brown. A pale green patch was present
below the eye; the spots on the posterior surfaces of the thighs were pale blue,
and those on the flanks were yellowish green. A calling male obtained 10
kilometers north-northwest of Chinajá (KU 55934) was reddish brown when
found at night; later the dorsal color changed to pale tan. A green patch below
the eye was persistent. Usually these frogs are green at night. The coloration
of an adult male (KU 87201) from 11 kilometers north of Vista Hermosa,
Oaxaca, México, was typical: "When calling dorsum pale green; later changed
to dull olive-green. Flanks dark brown with pale blue spots in axilla and
groin and bluish white flecks on mid-flank. Anterior and posterior surfaces of
thighs, inner surfaces of shanks, and median dorsal surfaces of tarsi dark brown
with blue spots. Canthal and postorbital stripes dark chocolate brown; labial
stripe creamy white. Forearm, tarsal, and anal stripes pale cream-color.
Throat dark brown with yellow flecks; belly and ventral surfaces of limbs
creamy buff; webs pinkish tan; iris deep bronze, brown below pupil." (Duellman,
field notes, June 24, 1964.)
Some individuals have both green and brown coloration in life. An individual
obtained at night on the south slope of Volcán San Martin, Veracruz,
México, had a pale tan dorsum changing peripherally to pale green. The
dorsal markings were dark brown and dark olive-green.
In contrast to the color-changes noted above, the labial region below the
eye is always pale green, and pale spots are always present on the flanks and
thighs in adults. The iris is invariably golden or bronze above and darker,
usually brown, below. Minute black flecks are present on the iris, and in some
individuals these flecks are so numerous that the eye appears gray.
Recently metamorphosed young have pale tan flanks, and the posterior surfaces
of the thighs are orange-yellow; pale spots are absent. A juvenile (KU
55935) from Chinajá, Alta Verapaz, Guatemala, having a snout-vent length
of 35.0 mm. was pale yellowish tan above with olive-green markings; the
flanks were dark brown with pale blue spots, and the anterior and posterior
[Pg 306]
surfaces of the thighs were uniform bright tomato red. A juvenile (UMMZ
121298), 18.6 mm. in snout-vent length, from the southeast slope of Volcán
San Martín, Veracruz, México, had pale tan flanks lacking blue spots, but had
red thighs. Apparently the ontogenetic changes in coloration proceed as follows:
(1) flanks pale tan and thighs orange-yellow, both lacking spots, (2)
flanks pale tan and thighs red, both lacking spots, (3) flanks dark brown with
blue spots and thighs red, lacking spots, and (4) flanks and thighs dark brown,
both having pale blue spots.
Natural History.—Smilisca cyanosticta inhabits humid tropical forest and
cloud forest from the lowlands to elevations of about 1200 meters in Los Tuxtlas
and to about 900 meters in northern Oaxaca. In these moist environments
the frogs apparently are active throughout the year. Active individuals have
been obtained in January, July, and August in Los Tuxtlas, Veracruz, in June
and July in northern Oaxaca, in February and March at Chinajá, Guatemala,
and Taylor and Smith reported (1945:589) activity in May at Piedras Negras,
Guatemala. Calling males were observed as follows; in a rain barrel 11 kilometers
north of Vista Hermosa, Oaxaca, México, on June 23-28, 1964; in a quiet
pool in a stream 8 kilometers south of Yetla, Oaxaca, México, in July, 1963
(Dale L. Hoyt, personal communication); in and near springs flowing into a
stream at Dos Amates, Veracruz, México, on August 4, 1959 (Douglas Robinson,
personal communication); and in a water-filled depression in a log 10
kilometers west-northwest of Chinajá, Guatemala, on March 13, 1960. Taylor
and Smith (1945:589) reported that individuals were found at night on the
ground at the edge of temporary pools at Piedras Negras, Guatemala, on May
28-29, 1939. A clasping pair was found on a rock at the edge of a small
stream on the south slope of Volcán San Martín, Veracruz, México, on July 11,
1959 (Douglas Robinson, personal communication).
Only one individual has been observed in a tree at night. In the daytime,
individuals were found in elephant ear plants (Xanthosoma) at Chinajá,
Guatemala.
The breeding call consists of one or two moderately short notes that are
lower pitched than those of S. baudini, but higher pitched than those of S.
phaeota. Each note has a duration of 0.25 to 0.45 seconds and is repeated at
intervals of one-half minute to several minutes. Each note is a vibrant
"waunk," having 110 to 180 pulses per second and dominant frequency of
1600 to 2100 cycles per second (Pl. 10B).
Apparently the eggs are deposited as loose clumps in the water. In eggs
in the yolk plug stage of development, the diameter of the embryo is about
2.3 mm.; that of the outer envelope is 4.0 mm. Hatchling tadpoles have total
lengths of 5.8 to 6.5 mm. and body lengths of 2.8 to 3.1 mm. The external
gills are moderately long, slender, and filamentous; the yolk sac is still moderately
large. The body and anterior part of the caudal musculature are dark
brown; posteriorly the caudal musculature is pale brown. The caudal fins are
creamy tan. The oral discs are large and ovoid. The growth of the tadpole
is summarized in Table 10.
A typical tadpole in stage 30 of development (KU 87652 from 11 km.
N Vista Hermosa, Oaxaca, México) can be described as follows:
Body length 9.5 mm.; tail length 15.5 mm.; total length 25.0 mm.; body
slightly wider than deep; snout rounded laterally, broadly ovoid dorsally; eyes
widely separated, directed dorsolaterally; nostril about midway between eye
[Pg 307]
and tip of snout; mouth anteroventral; spiracle sinistral, slightly posterior to
midpoint of body and slightly below midline; anal tube dextral; caudal
musculature slender, barely curved upward distally; dorsal fin not extending
onto body, depth of dorsal fin slightly more than that of ventral fin on mid-length
of tail; dorsal part of body dark brown; ventral surfaces transparent,
lacking pigment; posterior edge of body pale cream-color; caudal musculature
creamy white with interconnected brown spots; caudal fins transparent with
small brown blotches on dorsal fin and posterior half of ventral fin; iris coppery
bronze in life (Fig. 12). Mouth small, median part of upper lip bare; rest
of mouth bordered by single row of bluntly rounded papillae; lateral fold
present; tooth rows 2/3; all tooth-rows approximately equal in length; second
upper row broadly interrupted medially; other rows complete; upper beak
moderately deep, forming broad arch with slender lateral processes; lower beak
slender, broadly V-shaped; both beaks finely serrate (Fig. 15C).
All tadpoles having fully developed mouth parts have 2/3 tooth rows. Little
variation is noticeable in coloration. In many specimens the posterior edge
of the body is dark brown instead of pale cream-color. Mottling is rather
dense on the caudal fins in all specimens; in some individuals pigment is concentrated
along the anterior one-third of the lateral groove. In life the body
is dark brown with greenish gold flecks ventrally; the caudal musculature is
gray.
In each of two recently metamorphosed young the snout-vent length is 14.0
mm. Coloration of young in life (KU 87653 from 11 km. N Vista Hermosa,
Oaxaca, México): "Dorsum pale tan with dark brown markings. Thighs
orange-yellow; labial stripe white; iris bronze" (Duellman, field notes, July 10,
1964.)
Remarks.—Smith (1953:150) named cyanosticta as a subspecies of Hyla
phaeota. The differences in cranial characters and certain external characters
between phaeota and cyanosticta indicate that they are distinct species. Furthermore,
a gap of about 350 kilometers exists between the known geographic
ranges of the two kinds.
Distribution.—Smilisca cyanosticta inhabits wet forests on the Atlantic slope
of southern México and northern Central America from northern Oaxaca and
southern Veracruz through northern Chiapas in México and into El Petén and
northern Alta Verapaz in Guatemala (Fig. 2). Apparently the range is discontinuous,
for in southern México the species is found in cloud forest at
elevations of 830 to 900 meters on the northern slopes of the Sierra de Juárez.
In the Sierra de Los Tuxtlas in southern Veracruz the species is found in wet
forest at elevations of 300 to 1200 meters, but is absent in the intervening
lowlands characterized by drier forest. In the west forests of northern Alta
Verapaz and El Petén, Guatemala, the species is found at low elevations.
Specimens examined.—78, as follows: Mexico: Chiapas: Monte Libano,
MCZ 28271-9; 8 km. N San Fernando, 24 km. NE Tuxtla Gutierrez, UIMNH
41588. Oaxaca: 11 km. N Vista Hermosa, KU 84918-20 (skeletons), 87198-212,
87647 (eggs), 87648-52 (tadpoles), 87653 (young), UIMNH 57199-201; 8 km.
S Yetla, KU 87213, UMMZ 124838 (8). Veracruz: Coyame, UMMZ 111459-60;
between Coyame and Tebanco, UMMZ 121198; Dos Amates, UMMZ
121297; between Laguna de Catemaco and Volcán San Martín, UMMZ
121200; Volcán San Martín, UIMNH 35403-4, 35408-12, UMMZ 118163; SE
slope Volcán San Martín, UMMZ 121199, 121295 (2), 121296, 121298.
Guatemala: Alta Verapaz: Chinajá, KU 55935-7, 55938 (skeleton). El
[Pg 308]
Petén: 10 km. NNW Chinajá (Alta Verapaz), KU 55934; Piedras Negras,
CNHM 99006-7, 99227, UIMNH 28853, USNM 111139-41, 111143-7; 8 km.
S Piedras Negras, CNHM 99008; Semicoch, USNM 35907.
and Smilisca phaeota (circles).
Smilisca phaeota (Cope)
[Holotype.—USNM 4347 from Turbo, Colombia; J. Cassin collector].
Boulenger, Catalogue Batrachia Salientia in British Museum, p. 402,
Feb. 1, 1882. Werner, Sitzungsb. Akad. Wiss. München, 27:215, 1897.
Günther, Biologia Centrali-Americana: Reptilia and Batrachia, p. 269,
Sept. 1901. Nieden, Das Tierreich, Amphibia, Anura I, p. 261, June
1923. Dunn, Occas. Papers Boston Soc. Nat. Hist., 5:413, Oct. 10, 1931.
Gaige, Hartweg, and Stuart, Occas. Papers Mus. Zool. Univ. Michigan,
357:4, Oct. 26, 1937. Cooper, Copeia, 2:122, June 30, 1944. Breder,
Bull. Amer. Mus. Nat. Hist., 86(6):416, Aug. 26, 1946. Smith and
Taylor, Bull. U.S. Natl. Mus., 194:88, June 17, 1948; Univ. Kansas
Sci. Bull, 33:364, March 20, 1950. Taylor, Univ. Kansas Sci. Bull.,
35(1):837, July 1, 1952. Brattstrom and Howell, Herpetologica, 10:117,
[Pg 309]
Aug. 1, 1954. Goin, Herpetologica, 14:120, July 23, 1958. Cochran,
Bull. U.S. Natl. Mus., 220:57, 1961.
[Holotype.—ZMB 4913 from "region of Bogotá," Colombia]; Monats.
Konigl. Akad. Wissen. Berlin, p. 618, Oct. 16, 1873. Boulenger, Catalogue
Batrachia and Salientia in British Museum, p. 397, Feb. 1, 1882.
129:11, Jan. 25, 1923 [Holotype.—MCZ 8539 from Río Esnápe, Sambú
Valley, Darién, Panamá; Barbour and Brooks collectors]. Barbour and
Loveridge, Bull. Mus. Comp. Zool. Harvard, 69:278, June, 1929.
and Smith, Herpetologica, 16:103, June 17, 1960.
Dec. 30, 1960.
Diagnosis.—Size large ([M] 65 mm., [F] 78 mm.); skull as long as wide, lacking
frontoparietal fontanelle; large supraorbital flanges having straight edges
and extending posterolaterally; large squamosal not in contact with maxillary;
tarsal fold moderately wide, full length of tarsus; inner metatarsal tubercle
moderately large, low, flat, elliptical; hind limbs relatively long, tibia averaging
more than 53 per cent of snout-vent length; labial stripe silvery white; lips
lacking vertical bars; loreal region pale green; dark brown or black tympanic
mark dispersing into brown venated pattern on flanks; posterior surfaces of
thighs pale brown, with or without darker flecks or small cream-colored flecks.
(Foregoing combination of characters distinguishing S. phaeota from any other
species in genus.)
| Table 2.—Geographic Variation in Size and Proportions in Males of Smilisca phaeota. (Means in Parentheses Below Observed Ranges; Data Based of Ten Specimens From Each Locality.) | |||
| Locality | Snout-vent length | Head width/ snout-vent length | Interorbital distance/ head width |
| Bonanza, Nicaragua | 40.8-47.7 | 34.1-38.0 | 31.0-36.1 |
| (43.7) | (36.3) | (35.4) | |
| Heredia Prov., Costa Rica | 46.3-59.0 | 32.5-36.0 | 30.5-39.6 |
| (51.7) | (35.0) | (34.7) | |
| Puntarenas Prov., Costa Rica | 53.6-64.9 | 32.6-36.1 | 31.0-38.0 |
| (61.4) | (34.5) | (34.4) | |
| Canal Zone, Panamá | 52.4-65.5 | 33.5-37.6 | 31.3-37.2 |
| (56.5) | (35.6) | (34.7) | |
| Río Quesada, Colombia | 52.6-61.0 | 33.1-37.1 | 30.1-33.9 |
| (56.0) | (35.0) | (32.1) | |
Description and Variation.—For the purposes of analyzing geographic variation
in size and proportions, measurements were taken on ten adult males from
each of five samples throughout the range of the species. Aside from the data
summarized in Table 2, the average ratio of tibia length to snout-vent length
is noticeably less in Colombian specimens (53.4 per cent, as compared with
54.8 to 57.8 per cent in the other samples) and the ratio of head length to
[Pg 310]
snout-vent length is noticeably less in Costa Rican specimens (33.5 per cent
as compared with 34.9 to 35.1 per cent in the other samples). Also, specimens
from Heredia Province, Costa Rica, have a relatively smaller tympanum (62.7
to 80.4 [mean 68.4] per cent of the diameter of the eye, as compared with
means of 74.0 to 77.9 per cent in the other samples).
Two populations are distinctive as regards the size of adult males. Specimens
from the northern Caribbean lowlands of Nicaragua (Bonanza, the
northernmost locality for the species) are remarkably small. Males having
snout-vent lengths of between 40 and 43 mm. were breeding; the largest male
found had a snout-vent length of 47.7 mm. The other extreme in size is attained
in specimens from the Pacific lowlands of eastern Costa Rica and western
Panamá, where most breeding males have snout-vent lengths of more than
55 mm.; the largest male had a snout-vent length of 64.9 mm.
The rather striking differences in size among certain samples and the minor
differences in proportions among other samples show no geographic trends.
Instead, the variations apparently are random among the samples. The data
presented here possibly are the results of inadequate sampling, but more likely
reflect actual differences in the populations.
The dorsal ground color of Smilisca phaeota is pale green to tan; the venter
is creamy white. The dorsum is variously marked with dark olive-green or
dark brown spots or blotches (Pl. 6C). A dark interorbital bar is usually
present. Usually a large dark dorsal mark extends from the occiput to the
sacral region, but in many individuals this blotch is replaced by two or three
dark marks. The dorsal markings are irregular in shape and do not tend to
form transverse bands or longitudinal bars. The hind limbs are marked by
dark transverse bands, usually four or five on the thigh, five or six on the shank,
and four on the tarsus. Two or three narrow bands are usually present on the
proximal part of the fourth toe. The webbing on the feet is brown. The
loreal region is pale green, bordered above by a narrow dark brown canthal
stripe extending from the nostril to the orbit. The upper lip is silvery white.
A broad dark brown or black mark extends posteriorly from the orbit, encompassing
the tympanum, to a point above the insertion of the forelimb. The
flanks are pale green or pale tan and marked with a fine dark brown or black
venation. The anterior surfaces of the thighs usually are pale brown or grayish
tan, sometimes having small, indistinct darker flecks. The posterior surfaces
of the thighs are similarly colored, but in most specimens small but distinct
dark flecks are present; in some specimens small cream-colored spots are also
present on the posterior surfaces of the thighs. A distinct, narrow creamy white
anal stripe usually is present. A distinct white stripe is present on the
outer edge of the tarsus and fifth toe; on the tarsus the white stripe is bordered
below by dark brown. A white stripe also is present on the outer edge of the
forearm and fourth finger. In breeding males the throat is dark gray.
Little geographic variation in color or pattern is evident. Few, if any,
specimens from the Pacific lowlands of South America are green in life. (We
have seen no living individuals from South America.) Some living individuals
from Costa Rica and all those seen alive from Nicaragua have a tint of pale
blue on the flanks. In some specimens the dorsal pattern is so faint as to be
barely discernible, whereas in most specimens the pattern is bold.
The coloration in the living frogs is highly variable due to extreme metachrosis.
Individuals of this species are capable of changing the dorsal coloration
[Pg 311]
from green to brown in a short period of time. Both green and brown
individuals have been found active at night. Usually those individuals found
hiding by day are brown. One individual from Finca La Sumbadora, Panamá
(now KU 91914), was kept alive in the laboratory for nearly one month. This
individual usually was pale green with tan dorsal markings at night and tan
with pale green markings by day. On occasion the pale green dorsal markings
were boldly outlined by bright dark green.
In living individuals from throughout the range of the species the iris
is a bronze color, darkest medially with fine black reticulations.
Natural History.—Smilisca phaeota inhabits humid lowland tropical forest
and seldom ascends the foothills to more than 1,000 meters. The rather
equable climatic conditions, especially more or less evenly distributed rainfall
throughout the year, permit this frog to be active most of the year. Dunn
(1931:413) reported males calling on Barro Colorado Island, Panamá, in February
and in July, and Breder (1946:416) noted calling individuals in the
Chucanaque drainage of Darién, Panamá in January, March, July, August and
October and in Costa Rica in April through August inclusively. Calling males
were found at Bonanza, Nicaragua in March and in July.
At all times of year the usual daytime retreats for these frogs are near water;
the frogs have been found in elephant ear plants (Xanthosoma) and in bromeliads;
occasional individuals have been found sitting on shaded branches of
bushes and trees. None has been observed on the ground or beneath ground-cover
by day.
The length of the breeding season cannot be determined definitely. The
earliest date on which eggs have been found is May 23; Gaige, Hartweg, and
Stuart (1937:5) reported a gravid female taken at El Recreo, Nicaragua, in
September, and we have a gravid female taken at Almirante, Panamá, in
March.
Males usually call from secluded spots at the edge of water. All calling
males that we observed were on the ground within a few centimeters of the
water. The males usually are hidden beneath an overhanging leaf or some
other cover; they definitely do not sit in the open like Smilisca baudini. Most
calling males and clasping pairs have been found at the edges of small pools
or shallow ditches, although occasional individuals are found at the edges of
large ponds or streams.
The breeding call consists of one or two moderately short, low-pitched notes
(duration 0.33 to 0.42 seconds), repeated at intervals of about 20 seconds to
several minutes. Each note is a low, vibrant "wauk," having 100 to 130 pulses
per second and a dominant frequency of 330 to 420 cycles per second (Pl. 10C).
The eggs are deposited in loose clumps amidst vegetation in the water.
Hatchling tadpoles have total lengths of 8.7 to 10.6 mm., and body lengths
of 4.1 to 4.5 mm. The external gills are long and filamentous, and the yolk
sac is large. The head and caudal musculature are dark brownish black, and
the caudal fins are gray. The oral discs are large and roughly circular. The
growth and development of the tadpoles are summarized in table 11 and
figure 16.
A typical tadpole in stage 30 of development (KU 68482 from the Río
Chitaría, Cartago Province, Costa Rica) may be described as follows: body
length 9.7 mm.; tail length 14.6 mm.; total length 24.3 mm.; body as wide
as deep; snout rounded dorsally and laterally; eyes widely separated, directed
[Pg 312]
dorsolaterally; nostril about midway between eye and tip of snout; mouth
anteroventral; spiracle sinistral, about midway on length of body and slightly
below midline; anal tube dextral; caudal musculature slender, curved upward
distally; dorsal fin extending onto body; depth of dorsal fin slightly less than
that of ventral fin at mid-length of tail; dorsal part of body pale brown; ventral
surfaces transparent with scattered pigment; pale cream-colored, crescent-shaped
mark on posterior edge of body; caudal musculature pale creamy tan
with scattered pale brown spots; caudal fins transparent with scattered small
brown blotches on dorsal and ventral fins; iris pale bronze in life (Fig. 13);
mouth small; median part of upper lip bare; rest of mouth bordered by one
row of pointed papillae; lateral fold present; tooth-rows 2/3, first upper row
longest; second upper row slightly shorter, broadly interrupted medially; three
lower rows complete, equal in length, slightly shorter than second upper row;
upper beak moderately deep, forming broad arch with slender lateral processes;
lower beak slender, broadly V-shaped; both beaks serrate (Fig. 15E).
In tadpoles having fully developed mouthparts the tooth-row formula of
2/3 is invariable. The pale crescent-shaped mark on the posterior part of the
body curves anterodorsally on the dorsal surface of the body. These marks
in dorsal view give the appearance of a pair of short, pale stripes on the posterior
part of the body. Most specimens from Costa Rica have the pale coloration
like that described above, but some individuals (notable KU 87683 from
Guápiles, Costa Rica, KU 87707 from Finca Tepeyac, Nicaragua, and KU 87708
from Bonanza, Nicaragua) have much more pigment. In these specimens
the same color pattern obtains as in the pallid individuals, but the pigmentation
is dense. This is especially noticeable on the tail.
Recently metamorphosed young have snout-vent lengths of 12.7 to 16.7 mm.
(average, 14.3 mm. in eleven specimens). Coloration of young in life (KU
68484 from Río Chitaría, Cartago Province, Costa Rica): "Dorsum pale tan;
side of head and flanks darker brown, separated from tan dorsum by an
indistinct cream stripe. Limbs pale yellow; thighs flecked with brown; shank
and tarsus yellowish tan with indistinct brown bars. Soles of feet brown. Belly
white; throat dusty cream flecked with silvery white. Upper lip silvery white.
Iris bright gold with black flecks. Heels, tarsal and anal stripes white" (Duellman,
field notes, May 23, 1961).
Remarks.—Peters (1863:463) named Hyla labialis from the "region of
Bogotá, Colombia", but in 1873 regarded his new species as identical with
Hyla phaeota Cope, 1862, from Turbo, Colombia. The name Hyla labialis
has been used for frogs from the northern Andes in Colombia (see Dunn,
1944:72, and Stebbins and Hendrickson, 1959:522, for discussion of nomenclature).
Rivero (1961:131) used the name Hyla vilsoniana Cope, 1899, for
the frogs from the northern Andes previously referred to Hyla labialis. A
review of the nomenclature and taxonomy of these frogs, which superficially
resemble Smilisca but are unrelated, is beyond the scope of the present study.
Hyla baudini dolomedes Barbour, 1923, is based on a small Smilisca phaeota
(MCZ 8539) having a snout-vent length of 45.5 mm. Dunn (1931a:413)
placed dolomedes in the synonymy of Smilisca phaeota. We have examined
the holotype of dolomedes and agree with Dunn's assignment.
Smith (1953:150) described Hyla phaeota cyanosticta from Guatemala.
Our studies on the external morphology, coloration, and especially the cranial
osteology provide evidence that cyanosticta is a species distinct from phaeota.
[Pg 313]
Distribution.—Smilisca phaeota inhabits humid tropical forests from northeastern
Nicaragua southward on the Caribbean lowlands to elevations of about
1000 meters and on the Pacific lowlands of Costa Rica, exclusive of the arid
regions of Guanacaste, throughout the lowlands of Panamá, exclusive of the
savannas of the Pacific lowland and the Azuero Peninsula, and southward on
the Pacific slopes of South America through Colombia to west-central Ecuador;
also the valleys of the Río Cauca and Río Magdalena in Colombia (Fig. 2).
Specimens examined.—528, as follows: Nicaragua: Matagalpa: Finca
Tepeyac, 10 km. N, 9 km. E Matagalpa, KU 85439, 87707 (tadpoles); Matagalpa,
MCZ 3546-7, UMMZ 92367; 19 km. N Matagalpa, UMMZ 116495-6.
Zelaya: Bonanza, KU 84854-62, 84950-2 (skeletons), 85440-50, 87708-9 (tadpoles);
Cukra, AMNH 80618; Río Mico, 16 km. E Recreo, UMMZ 79711 (6),
79712 (4); junction Río Mico and Río Siguia, UMMZ 79713 (10); Río Siguia,
11 km. NW Rama, UMMZ 79714 (14), 79715 (11), 79716 (21), 79717, 79718 (3).
Costa Rica: Alajuela: Cinchona, KU 32255, 64286-8; 5 km. S Ciudad
Quesada, USC 8077; Laguna Monte Alegre, KU 64289-90; Las Playuelas, 11
km. S Los Chiles, USC 7216; San Carlos, USNM 29961.
Cartago: Moravia de Turrialba, KU 32212-47, 37133-5, 41093 (skeleton),
64280-1, USC 7243 (3); Peralta, KU 32271-2; Río Chitaría, 3 km. NNE Pavones,
KU 64273-9, 68477 (eggs), 68478-83 (tadpoles), 68484 (young); Río
Reventazón, MCZ 29196-203, UMMZ 117677 (9); Turrialba, KU 25720-2,
32209-11, 32266-8, 32273-4, 37136-67, 41090-2 (skeletons), 64270-2, MCZ
29221, 29222 (tadpoles), 29269-70, USNM 29934.
Guanacaste: Tilarán, KU 36805-7; 8 km. NE Tilarán, KU 36803-4.
Heredia: Barranca del Río Sarapiquí below Isla Bonita, KU 64282-3; Cariblanco,
KU 32256-60, 41094 (skeleton), 64284, MCZ 7967; Isla Bonita, KU
32250-4; 4.2 km. W Puerto Viejo, KU 64285, 68485; 7.5 km. W Puerto Viejo,
KU 68486; 1 km. S Puerto Viejo, KU 86518.
Limón: Bambú, USC 7182 (4); Batán, UMMZ 118582; Coén, MCZ 9825;
La Lola, KU 32262-4, UF 4029, UMMZ 117678 (3); Los Diamantes, CNHM
101295-8, KU 25723-4, 32265, 64267-9; Pandora, UMMZ 122650 (2), USC
7188 (3), 7190; Puerto Limón, KU 32261; Río Larí at Río Dipari, 21 km. SW
Amubre, USC 7177; Río Toro Amarillo, 7 km. W Guápiles, KU 86519, 87683
(tadpoles); Suretka, KU 36808-10, 37168.
Puntarenas: Agua Buena, KU 36790; 1.6 km. E Buenos Aires, UMMZ
117578; 3 km. NW Buenos Aires, KU 64304; 4 km. N, 15 km. W Dominical,
KU 68491-2 (tadpoles); Esparta, MCZ 8029-30, 8032; Golfito, KU 32270; 6
km. E Golfito, KU 84999-500 (skeletons); Gromaco, UMMZ 123677 (4); Palmar,
KU 32269; 4 km. ESE Palmar Sur, KU 64305-6; 5.6 km. SE Palmar Sur,
KU 68489 (tadpoles); 7.0 km. SE Palmar Sur, KU 68490 (young); 8.5 km. SE
Piedras Blancas, KU 64292-303; Quebrada Boruca, 22 km. E Palmar Norte,
KU 64291; Rincón, "Camp Seattle," Peninsula de Osa, UMMZ 123676 (3),
USC 7254; Río Ferruviosa, 7 km. S Rincón, USC 7235; 1.6 km. WNW Villa
Neily, KU 68493 (young), 68494 (tadpoles).
San José: San Isidro el General, KU 32249, UMMZ 75025; 10 km. N San
Isidro el General, MCZ 29099-103; 13 km. WSW San Isidro el General, KU
86517; 15 km. WSW San Isidro el General, KU 68487 (tadpoles), 68488
(young), 68495 (young); 20 km. WSW San Isidro el General, KU 32248.
Panama: No province: Cano Saddle, USNM 69588; Punta de Pena,
USNM 38733; Quipo, AMNH 18925-6. Bocas del Toro: Almirante, KU
80080, 91835-6; 1.6 km. W Almirante, KU 91837; 3 km. W Almirante, KU
91824 (skeleton), 91838-43, 91906-7; 11 km. NW Almirante, CNHM 67853-61;
13 km. W Almirante, KU 91825-7 (skeletons), 91844-9; Fish Creek, KU 92329;
Isla Popa, KU 91850-1. Canal Zone: Barro Colorado Island, CNHM 6007,
13316, 13325, 13331, 13360-2, 13377-8, MCZ 24191-5, UF 7523, UMMZ
63547-60, 64457, 69497 (3); 3.7 km. W Cocoli, KU 67916; Fort Sherman,
MCZ 10139; Gatun, MCZ 35644; Junction roads C25B and C16, TNHC 23839;
[Pg 314]
Madden Forest Preserve, TNHC 23837-8. Coclé: El Valle, KU 77521-4,
77649 (tadpole), TNHC 23369. Comarca del Barú: Progreso, UMMZ 61085-9.
Colón: Achiote, KU 77516-20, 77648 (young); Río Candelaria, CNHM 67851-2.
Darién: Río Esnápe, Sambú Valley, MCZ 8539; Río Sucubti, Chalichiman's
Creek, AMNH 40512; Camp Creek, AMNH 40758-9; Camp Creek,
Camp Townsend, AMNH 40988. Panamá: NW slope Cerro Prominente, KU
80459; Finca La Sumbadora, KU 91914 (skeleton). Chiriquí: 2 km. W Concepción,
AMNH 68910.
Columbia: Antioquia: Puerto Berrio, CNHM 30805 (Goin); Turbo, USNM
39899. Caldas: Pueblorrica, Santa Cecilia, CNHM 54768-71 (Goin). Choco:
No specific locality, AMNH 3984-6; Andagoya, BMNH 1915.10.21. 69-70,
CNHM 81857 (Goin); Golfo de Urabá, CNHM 63881 (Goin); Peña Lisa,
Condoto, BMNH 1913.11.12. 118-125, 1913.11.12. 137-146 (Goin); Pizarro,
CNHM 4451-3, 4455-61 (Goin); Río San Juan, Playa del Oro, CNHM 54772
(Goin); Río Quesada, AMNH 13615-77; 37 km. up Río Puné, AMNH 13688;
48 km. up Río Puné, AMNH 13689. Narino: Tumaco, Río Rosario, CJG
2310-13 (Goin). Valle: Buenaventura, BMNH 1895.11.16.82 (Goin); Raposa,
WAT 166, 346-47, 388 (Goin); Río Calima above Córdoba, CJG 2249-57
(Goin).
Ecuador: No province: Bulun, AMNH 10620. Esmeraldas: Cachabé,
AMNH 10625-8; Río Capayas, CNHM 35712; Río Sapaya, UMMZ 58910 (5);
Salidero, AMNH 10623-4; San Javier, AMNH 10618. Guayas: Hacienda
Balao Chico, UMMZ 123904. Imbabura: Pambelar, AMNH 10629, 10631.
Pichincha: Hacienda Espinosa, 9 km. W Santo Domingo de los Colorados,
KU 40220.
Smilisca puma (Cope), new combination
13735 from Nicaragua; J. F. Moser collector]. Günther, Biologia Centrali-Americana:
Reptilia and Batrachia, p. 270, Sept., 1901. Nieden, Das
Tierreich, Amphibia, Anura I, p. 251, June, 1923. Cochran, Bull. U. S.
Natl. Mus., 220:58, 1961.
[Holotype.—KU 30302 from Batán, Limón, Costa Rica, Edward H. Taylor
collector]; Univ. Kansas Sci. Bull., 36(1):626, June 1, 1954. Duellman
and Berg, Univ. Kansas Publ. Mus. Nat. Hist., 15:194, Oct. 26, 1962.
4:303, Dec. 30, 1960.
Diagnosis.—Size small ([M] 38.0 mm., [F] 46.0 mm.), differing from other
species in the genus by the following combination of characters: skull about
as long as broad; frontoparietal fontanelle keyhole-shaped; supraorbital flanges
absent; squamosal small, not in contact with maxillary; bony portion of ethmoid
terminating at anterior edge of orbit; tarsal fold weak, two-thirds length of
tarsus; inner metatarsal tubercle small, low, flat, elliptical; snout rounded in
dorsal profile; lips thin and flaring; fingers having only vestige of web; toes one-half
webbed; diameter of tympanum about two-thirds that of eye; narrow
labial stripe white; pair of dark brown (sometimes interconnected) stripes on
tan dorsum; no blue spots on flanks or thighs; vocal sac in breeding males
pale brown. (Foregoing combination of characters distinguishing S. puma
from other species in genus.)
Description and variation.—Ten breeding males from the vicinity of Puerto
Viejo, Heredia Province, Costa Rica, have snout-vent lengths of 32.5 to 37.9
mm. (34.8 mm.). In these specimens, the length of the tibia to the snout-vent
length is 0.48 to 0.53 (0.51), and the tympanum/eye ratio is 0.52 to 0.72
(0.65). Seven females have snout-vent lengths of 40.8 to 45.8 mm. (43.9 mm.).
[Pg 315]
No individual has more than a vestige of a web between the second and third
and fourth fingers. None has a web between the first and second fingers.
Breeding males lack nuptial excrescences on the thumbs. The vocal sac is
moderately large and bilobed.
In preserved specimens the dorsal ground color varies from yellowish tan
to grayish brown. All specimens have dark brown dorsal markings in the form
of a pair of dorsal stripes, variously modified (Pl. 7A). In some specimens,
such as KU 91716, the stripes are discrete and extend from the postorbital
region nearly to the vent. In most specimens the stripes are connected by a
transverse mark in the scapular region and in many others also by a transverse
mark in the sacral region. In some specimens the stripes are fragmented
posteriorly; fragmentation is extreme in KU 30300, in which the dorsal pattern
consists of two series of dark longitudinal dashes. The other extreme is
a nearly complete fusion of the stripes, as in KU 91714. A dark brown interorbital
bar usually extends onto the eyelids, but in some specimens this is
reduced to a short V-shaped mark or small spot between the eyes. There is
no dark post-tympanic mark, but dark brown pigment forms a venated pattern
from the axilla to the mid-flank; the inguinal region is white, finely mottled with
dark brown. The dorsal surfaces of the hind limbs are colored like the body
and have two or three dark brown transverse marks on the thighs, three to five
marks on the shanks, and one or two marks or irregularly arranged dark flecks
on the tarsi. The anterior and posterior surfaces of the thighs are pale tan to
brown. The webbing of the feet is tan to grayish brown. A narrow white
labial stripe, white anal stripe, and narrow white stripes on the tarsi and outer
edges of the forelimbs are invariably present. The ventral surfaces are creamy
white.
In life the dorsum is tan or pale brown with dark brown markings. Some
individuals have scattered metallic green flecks on the dorsum. The flanks
are mottled dark brown and creamy white. The posterior surfaces of the
thighs are dark brown. The vocal sacs are grayish brown, and the iris is a
deep bronze color.
Natural History.—Smilisca puma inhabits humid lowland tropical forests
having more or less evenly distributed rainfall throughout the year. The
equable climatic conditions seemingly permit these frogs to be active throughout
most of the year. Taylor (1952:846) found calling males at Batán, Costa
Rica, on July 20, 1951. We found the species breeding near Puerto Viejo,
Costa Rica, on February 19, June 18, July 13, and July 31. Specimens of
calling males from Costa Rica in the collection at the University of Southern
California were obtained in February at La Fortuna, on August 22 at Los
Diamantes, on August 30 at Jabillos, and on September 5 at La Lola. Gravid
females were collected in June, July and August.
Males call from shallow water. All breeding congregations of this species
that we have found were in a grassy marsh, 7.5 kilometers west of Puerto
Viejo, Costa Rica. Tadpoles were found in water-filled depressions in the
marsh at night. When first observed, tadpoles were near the surface of the
water; they responded to light by quickly taking refuge in the dense grass.
No tadpoles were observed by day.
The breeding call consists of a low squawk, usually followed by a series
of one or more rattling secondary notes (duration of primary notes, 0.06-0.35
[Pg 316]
seconds; of secondary notes, 0.10 to 0.47 seconds), repeated at intervals of
5 to 55 seconds. The primary notes have 187 to 240 pulses per second and
major frequencies of about 740 to 1870 cycles per second (Pl. 11A).
Only six tadpoles are available for study. Four of them in stage 34 of
development have body lengths of 9.0 to 9.5 mm., tail lengths of 14.0 to 15.0
mm., and total lengths of 23.0 to 24.5 mm. One tadpole in stage 38 and one
in stage 40 have total lengths of 31.0 mm. A typical tadpole in stage 34 of
development (KU 91807 from 7.5 km. W Puerto Viejo, Heredia Province,
Costa Rica) has a body length of 9.5 mm., tail length of 15.0 mm., and total
length of 24.5 mm.; body about three-fourths as deep as wide; snout rounded
dorsally and laterally; eyes widely separated, directed dorsolaterally; nostril
about midway between eye and tip of snout; mouth anteroventral; spiracle
sinistral, about two-thirds distance from snout to posterior end of body and
slightly below midline; anal tube dextral; caudal musculature slender, barely
curved upward distally; dorsal fin extending onto body; at mid-length of tail,
depth of caudal musculature equal to that of dorsal fin and ventral fin; body
grayish brown, palest ventrally; caudal musculature pale creamy yellow with
bold gray reticulations; caudal fins transparent with gray reticulations anteriorly
and black flecks posteriorly on both fins (Fig. 14A). Median part of upper
lip bare; rest of mouth bordered by two rows of short blunt papillae; lateral
fold present; tooth-rows 2/3; upper rows equal in length; second upper row
broadly interrupted medially; three lower rows complete, first and second
rows equal in length, slightly shorter than upper rows; third lower row noticeably
shorter; upper beak shallow, forming broad, continuous arch with slender
lateral processes; lower beak slender, broadly V-shaped, both beaks finely
serrate (Fig. 15B).
All six tadpoles are colored alike, except that in the larger specimens scattered
white flecks are present on the ventral surface of the body, and the dark
reticulations continue farther posteriorly on the caudal fins than in the smaller
tadpoles. In two specimens the third lower tooth-row is only about one-half
the length of the other lower rows, and in one specimen the second lower
tooth-row is shorter than the first. Coloration of tadpoles in life: "Body olive-brown
with silvery green flecks laterally. Caudal musculature olive-brown with
greenish tan flecks. Fins brown with greenish gold flecks. Iris deep bronze."
(Duellman, field notes, February 19, 1965).
One recently metamorphosed young (KU 91808) has a snout-vent length
of 12.4 mm. In life this frog had a pale tan dorsum with dark brown markings,
yellowish tan posterior surfaces of thighs, grayish brown throat, and
bronze iris.
Remarks.—The identity of Cope's Hyla puma has not been known. The
name has appeared in various compilations, but no workers have referred any
of their specimens to that species. Examination of the holotype (USNM
13735), an adult female, revealed the presence of the following combination
of characters: snout-vent length 45.8 mm., snout blunt above and rounded
laterally, nostrils close to tip of snout, lips thin and flaring, a vestige of a
web on the hands, feet about one-half webbed, tarsal fold weak and extending
about two-thirds length of tarsus, dorsal markings consisting of a faded dark
interorbital bar and a pair of faded longitudinal brown marks connected by
a transverse band in the scapular region. The type agrees well with specimens
of Smilisca wellmanorum (Taylor, 1952); the vestigial webbing on the
[Pg 317]
hands and the dorsal coloration are especially significant. Consequently, we
consider Hyla wellmanorum Taylor, 1952, to be a synonym of Hyla puma Cope,
1885. Cope gave only "Nicaragua" as the locality for Hyla puma. The specimen
was part of a collection received at the United States National Museum
from Lt. J. F. Moser. Among the species in the collection are Dentrobates
pumilio, Phyllomedusa helenae, Corythophanes cristatus, Pliocercus dimidatus,
Tretanorhinus nigroluteus, and others characteristically found on the Caribbean
lowlands of Central America. Thus, it seems reasonable to assume that
the type specimen of Hyla puma came from the Caribbean lowlands. Though
no other Nicaraguan specimens have been found by us, numerous specimens
are known from the Caribbean lowlands of Costa Rica.
Cochran (1961:58), in her catalogue of type specimens in the United
States National Museum, listed Hyla puma Cope, 1885, as a synonym of Hyla
molitor Schmidt, 1857. She made no qualifying statements. Schmidt (1858:246),
in his descriptions of the species in the year following his publication
of the names and Latin diagnoses, stated: "Dorsum uniformly gray, more
intensive on back, fading away laterally and on extremities; in every-day-life
this blue would be called Mueller's Blau. A delicately dotted black line runs
on the canthus rostralis from the opening of the nose to the corner of the eye.
In the armpits, on the flanks and the thighs two of our three specimens have
black marblings." [Free translation] Certainly on the basis of coloration
Hyla puma is distinctly different from Hyla molitor.
Distribution.—This species lives in the wet, forested regions of the Caribbean
lowlands of Costa Rica and presumably southern Nicaragua (Fig. 3).
All specimens are from low elevations; the highest known elevation for the
occurrence of this frog is 285 meters at Laguna Bonilla.
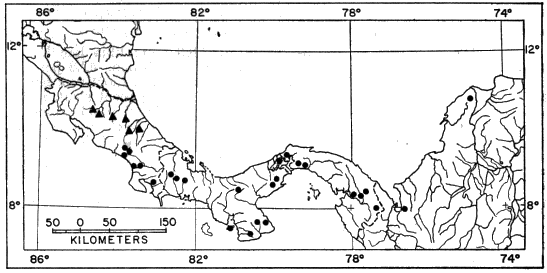
Smilisca sila (circles).
Specimens examined.—62, as follows: Nicaragua: No specific locality,
USNM 13735.
Costa Rica: Alajuela: Jabillos, 5 km. N Santa Clara, USC 8058 (6); 5
km. W La Fortuna, USC 8078 (2); Río La Fortuna at La Fortuna, USC 7151
(3). Cartago: Laguna Bonilla, tunnel camp near Peralta, KU 32171. Heredia:
Puerto Viejo, KU 86521; 5.9 km. W Puerto Viejo, KU 64307; 7.5 km. W Puerto
[Pg 318]
Viejo, KU 64308-10, 64311 (skeleton), 64312-15, 68635-6 (skeletons), 85001-2
(skeletons), 86520, 87770-1 (skeletons), 91709-16, 91791-2, 91807 (tadpoles),
91808 (young). Limon: Batán, KU 30300-2; La Lola, KU 32169, USC 141,
201, 8067; Los Diamantes, KU 32170, UMMZ 118470 (6), USC 212; 2.4 km.
E Los Diamantes, USC 8049 (5).
Smilisca sila new species
Dunn, Occas. Papers Boston Soc. Nat. Hist., 5:413, Oct. 10, 1931.
Schmidt, Smithsonian Misc. Coll., 89(1):6, March 16, 1933.
June 30, 1944. Breder, Bull. Amer. Mus. Nat. Hist., 86(8):417, Aug.
26, 1946.
1946.
Holotype.—Adult male, KU 91852 from a small stream at the north edge
of the village of El Volcán, Chiriquí Province, Panamá, elevation 1280 meters;
obtained on Feb. 5, 1965, by William E. Duellman.
Paratypes.—KU 91853-74, collected with the holotype.
Diagnosis.—Size moderate ([M] 45.0 mm., [F] 62.2 mm.); skull wider than
long, having large, ovoid frontoparietal fontanelle; supraorbital flanges absent;
squamosal small, not contacting maxillary; bony section of ethmoid extending
anteriorly between nasals; tarsal fold weak, full length of tarsus; inner metatarsal
tubercle low, flat, elliptical; lips thick, rounded, not flaring; fingers one-third
webbed; toes three-fourths webbed; diameter of tympanum about one-half
that of eye; margin of upper lip faintly marked by interrupted creamy
white stripe; dark spots on dorsum; pale flecks on flanks and posterior surfaces
of thighs; vocal sacs in breeding males dark brown. (Foregoing combination
of characters distinguishing S. sila from any other species in genus.)
Description of holotype.—Snout-vent length 36.6 mm.; tibia length 19.8
mm., 54.1 per cent of snout-vent length; foot length 15.5 mm., 42.3 per cent
of snout-vent length; head length 12.7 mm., 34.7 per cent of snout-vent length;
head width 13.3 mm., 36.8 per cent of snout-vent length; snout short, in lateral
profile truncate, only slightly rounded above, in dorsal profile rounded;
canthus rounded; loreal region noticeably concave; lips thick, rounded, not
flaring; nostrils not protuberant, directed laterally; internarial distance 3.0 mm.;
internarial area flat; top of head flat; interorbital distance 3.5 mm., 26.3 per
cent of head width; diameter of eye 4.2 mm., thrice distance (1.4 mm.) from
tympanum to eye, and half again distance (2.8 mm.) from orbit to nostril;
pupil horizontally ovoid; width of eyelid 2.8 mm., 21.1 per cent of head width;
dermal fold from posterior corner of orbit covering upper edge of tympanum
to point above insertion of forelimb; diameter of tympanum 2.3 mm., 54.7 per
cent of diameter of eye; no axillary membrane; arms moderately robust; weak
fold on wrist; faintly scalloped fold along ventrolateral margin of forearm;
fingers short, slender; fingers from shortest to longest, 1-2-4-3; vestige of web
between first and second fingers; others about two-fifths webbed; discs moderate,
diameter of that on third finger about one-third diameter of eye; triangular
outer palmar tubercle; elliptical inner palmar tubercle on base of
pollex; subarticular tubercles large, conical, none bifid; supernumerary tubercles
few, large, conical; brown nuptial excrescence on prepollex; heels overlap by
about one-fifth length of shank when hind limbs adpressed; tibiotarsal articulation
extending to nostril; tarsal fold weak, extending nearly full length of
tarsus; inner metatarsal tubercle elliptical, flat; outer metatarsal tubercle absent;
toes moderately long; toes from shortest to longest, 1-2-3-5-4, third and
fifth about equal in length; discs about same size as those on fingers; webbing
[Pg 319]
extending to middle of penultimate phalanx on all toes, except only to distal
end of antepenultimate phalanx of fourth toe; subarticular tubercles round;
supernumerary tubercles large, round, present only on proximal digits. Anal
opening directed posteriorly at level of upper edge of thighs; no noticeable
anal sheath; flat tubercles ventrolateral to anal opening large; skin of chest,
belly, and posterior surfaces of thighs granular; other surfaces smooth; tongue
broadly cordiform, shallowly notched posteriorly, and barely free behind;
vomerine teeth 4-4, situated on ventral surfaces of separated rounded prominences
between posterior margins of small, ovoid inner nares; vocal slits long,
each situated along inner margin of ramus; color (in preservative) pinkish tan
above with irregular olive-brown markings forming interconnected spots on
back; four bars on dorsal surface of each thigh; five bars on shank, and three
on tarsus; inguinal region white with black mottling; posterior surfaces of
thighs yellowish tan proximally, dark brown distally; margins of lips grayish
white with brown markings; ventral surfaces of hands and feet grayish brown;
belly and posterior part of throat creamy white; anterior part of throat brown.
Description and variation.—Ten breeding males from Finca La Sumbadora,
Panamá, have snout-vent lengths of 40.0 to 44.8 mm. (42.3 mm.). In these
specimens the tibia/snout-vent length ratio is 0.50 to 0.57 (0.54), and the
tympanum/eye ratio is 0.48 to 0.58 (0.53). There is a geographic gradient
in size; specimens from the western part of the range (southern Costa Rica)
are smaller than those from the eastern part of the range (eastern Panamá).
Five males from the Pacific lowlands of southern Costa Rica have snout-vent
lengths of 31.6 to 38.2 mm. (34.7 mm.); ten males from El Volcán, Chiriquí,
Panamá, 32.6 to 37.9 mm. (36.4 mm.), and eight males from Barro Colorado
Island, Canal Zone, 38.2 to 42.0 mm. (35.6 mm.). These are smaller than the
males from Finca La Sumbadora, which is east of the Canal Zone. Ten females
from El Volcán have snout-vent lengths of 44.2 to 55.6 mm. (49.2 mm.),
as compared 56.1 to 62.2 mm. (58.2 mm.) in three females from Finca
La Sumbadora.
Large females have scattered small tubercles on the head and back; tubercles
occur in males from Costa Rica and in some males from western Panamá.
The truncate snout is characteristic of both sexes.
The coloration of Smilisca sila consists of a gray, tan, or pale reddish brown
dorsal ground color and a creamy white venter. The dorsum is marked by
dark brown, olive-brown, or dark reddish brown spots or blotches (Pl. 7B).
Usually the blotches are discrete, but in some individuals they are interconnected
and form an irregular dark mark on the dorsum. There is no tendency
for the blotches to form transverse bars as in Smilisca sordida. In one specimen
(KU 80467) the blotches are fused and form two wide irregular longitudinal
stripes, as in Smilisca puma. In some females the dorsal markings are
reduced to a few small spots or are nearly absent (KU 92332), whereas in
other females the dorsal markings are bold. In one female (KU 91894) the
dorsal markings are narrowly bordered by pale blue, and numerous pale blue
flecks are present on the pale brown dorsum. In many individuals of both
sexes small white flecks are present on the dorsal surfaces.
Usually the flanks and posterior surfaces of the thighs have black mottling
enclosing pale blue spots and flecks, respectively. The dorsal surfaces of the
limbs are marked by dark brown transverse bars; usually three or four bars
are present on each forearm, thigh, and shank. The coloration of the flanks
and limbs varies geographically. Specimens from southern Costa Rica and
western Panamá have distinct bars on the limbs; the posterior surfaces of the
thighs have brown reticulations enclosing small blue flecks in specimens from
[Pg 320]
Costa Rica and bolder, black reticulations enclosing large pale blue spots in
specimens from western Panamá. In specimens from Costa Rica the flanks are
brown with pale blue flecks, whereas in those from Chiriquí, Panamá, the
flanks are pale blue with dark brown mottling in the inguinal region. Frogs
from El Valle and Cerro la Campana usually have distinct bars on the limbs;
the posterior surfaces of the thighs are colored as in frogs from Chiriquí, and
the inguinal region is pale blue with coarse brown mottling. Specimens from
Barro Colorado Island are marked like those from El Valle and Cerro la Campana,
except that on the posterior surfaces of the thighs fine black reticulations
enclose many dark blue spots. In specimens from Darién and from Panamá
Province east of the Canal Zone (Altos de Pacora, Cerro Jefe, Finca La Sumbadora,
and Río Pacora), the markings on the dorsal surfaces of the limbs are
indistinct or absent in males, but distinct in some females. Intense brown
and black pigment forms fine reticulations delimiting bold blue spots on the
flanks; this coloration extends to the axilla in many specimens. Fine black
reticulations enclose many dark blue spots on the posterior surfaces of the
thighs.
In females, the throat is creamy white; in some specimens scattered brown
flecks are present on the chin and throat. In breeding males the anterior part
of the throat is dark gray or dark brown.
The coloration in life is as variable as it is in preservative. In life the
holotype had a tan dorsum with dark olive-green irregular markings and small
green flecks. The limbs were tan with dark brown transverse bars. The flanks
were grayish tan anteriorly; the inguinal region and posterior surfaces of thighs
were blue with black mottling. The belly was creamy white, and the throat
was brown with creamy yellow flecks. The iris was a dull bronze color.
Among the paratypes, some individuals had green flecks, others did not. The
inguinal region and posterior surfaces of the thighs were pale blue, pale yellowish
green, or grayish tan with black mottling. The blue was most noticeable
in females.
Colors of a male from Finca La Sumbadora, Panamá, were described as
follows: "Dorsum olive-brown; irregular dark brown blotches, pale green
flecks, and raised creamy yellow spots on dorsal surfaces; belly creamy white;
throat grayish brown; undersides of limbs grayish tan; groin, anterior and
posterior surface of thigh, inner surface of shank, anterior edge of tarsus, and
proximal parts of third and fourth toes pale blue marbled with dark brown
and black; webbing brown; iris pale bronze, finely reticulated with black."
(Duellman, field notes, January 28, 1964.)
A female (now KU 91894) from Altos de Pacora, Panamá, was described
as follows: "An irregular dark brown, green-bordered figure on head and
back; dark brown, green-bordered bands on limbs—all on a lighter brown and
heavily green-spotted background. These markings are more vivid at night
than during the day. Lower sides, from midbody onto front of thighs and
rear of thighs onto venter of shanks to heels and thence dorsally onto basal
portions of toes heavily blue spotted on a light brown (front of thighs and
venter of shanks) to blackish brown background. Venter cream. Iris gray-brown,
finely veined with dark brown." (Charles W. Myers, field notes, December
14, 1964.) Note that in the earlier discussion of coloration of preserved
specimens, the green spots and borders have changed to pale blue after
six months in alcohol.
[Pg 321]
In living individuals from Costa Rica and Panamá west of the Canal Zone,
the blue coloration on the flanks and thighs is much less conspicuous than in
specimens from eastern Panamá. The color of the iris is variable, even in
frogs from one locality. The coloration of the iris in 13 living frogs (now
KU 92333-45) from Valle Hornito, Chiriquí, Panamá, was described as follows:
"Iris variable—from pale to dark brown; in a few the iris has a golden
cast to the brown; in a few others the lower half of the iris is pale gray with
the upper half being light brown." (Charles W. Myers, field notes, April 24,
1965).
Natural history.—Smilisca sila inhabits the Pacific slopes of lower Central
America where a pronounced dry season occurs. We have records of males
calling in December through May and also in August (latter date from El
Volcán, Chiriquí, Panamá). The breeding season seems to be correlated with
the time of the year when the water is clear and at a low level in the streams
where these frogs breed.
Males call from the edges of small, shallow streams, from rocks in the
streams, or less frequently from vegetation overhanging the streams. Females
are most frequently found on the banks of streams, and clasping pairs usually
are in shallow pools in streams. One individual was found in a bromeliad
about three meters above the ground in the daytime.
The breeding call consists of a low squawk, usually followed by a series
of one or more rattling secondary notes (duration of primary notes, 0.06 to
0.28 seconds; of secondary notes, 0.14 to 0.48 seconds), repeated at intervals
of 4 to 20 seconds. The primary notes have 97 to 120 pulses per second and
major frequencies of about 900 to 2220 cycles per second (Pl. 11B).
Eggs were obtained artificially in the field; the average length of ten
embryos in the neural groove stage is 2.4 mm., and the average diameter of
the outer envelope is 4.9 mm. Hatchlings have large, conical oral discs, heavy
gills, and a large amount of yolk; their average total length is 6.3 mm.
Tadpoles have been found in pools in clear streams; some tadpoles have
been observed to cling by their mouths to rocks in the stream; others were
found on the bottom where they seek refuge among pebbles or under rocks
and leaves. A complete developmental series of tadpoles is not available.
Eleven tadpoles in stage 25 of development have body lengths of 8.3 to 10.2
mm. (9.3 mm.), tail lengths of 17.3 to 21.0 mm. (18.8 mm.), and total lengths
of 25.9 to 31.0 mm. (28.1 mm.). One tadpole in stage 41 and one in stage 42
have body lengths of 11.5 and 12.5 mm., tail lengths of 27.2 and 29.5 mm.,
and total lengths of 38.7 and 42.0 mm., respectively. The snout-vent lengths
of two specimens in stage 43 and one in stage 45 are 12.7, 13.0, and 13.6 mm.,
respectively.
A typical tadpole in stage 25 of development (KU 80620 from Finca La
Sumbadora, Panamá) has a body length of 9.5 mm., tail length of 19.0 mm.,
and a total length of 28.5 mm.; body only slightly wider than deep, nearly
flat dorsally; snout broadly rounded in dorsal view, bluntly rounded in lateral
view; eyes widely separated, directed dorsolaterally; nostril slightly closer to
eye than to tip of snout; mouth ventral; spiracle sinistral, located about two-thirds
distance from snout to posterior edge of body; anal tube dextral; caudal
musculature moderately heavy, straight; dorsal fin not extending onto body;
fins deepest at about two-fifths length of tail, where depth of caudal musculature
about equal to depth of dorsal and depth of ventral fin; musculature
[Pg 322]
extending nearly to tip of tail; body dark grayish brown above and pale grayish
tan below with small dark brown spots dorsally and white flecks laterally;
caudal musculature pale tan with dark brown flecks over entire surface and
dark brown streaks on posterior one-half of ventral fin and on all of dorsal
fin (Fig. 14B). Median one-third of upper lip bare; rest of mouth bordered
by a single row of conical papillae; lateral fold present; tooth rows 2/3; upper
rows cone-shaped, about equal in length, broadly ∧-shaped; second upper
row narrowly interrupted medially; lower rows complete, about equal in
length, but slightly shorter than upper rows; upper beak moderately massive,
its inner surface forming a continuous arch with short lateral processes; lower
beak broadly ∨-shaped; both beaks finely serrate (Fig. 15D).
Tadpoles from El Volcán, Chiriquí (KU 91833), are more heavily pigmented
than those from Finca La Sombadora; the spots on the tail are larger.
In life these tadpoles had dark brownish black bodies with golden and green
lichenous flecks; the tail was tan with dark brown markings, and the iris was a
grayish bronze color. In life tadpoles from Finca La Sumbadora were olive-tan
above and dark gray with pale bluish gray irridescent spots ventrally. The
caudal musculature was creamy tan with brown flecks and streaks, and the
iris was pale bronze.
Metamorphosing young have been found on vegetation at the edge of
streams and have been raised in the laboratory. Seven recently metamorphosed
young have snout-vent lengths of 13.6 to 15.6 mm. (14.6 mm.). A
living juvenile (KU 91913) raised in the laboratory from a tadpole obtained
at Finca La Sumbadora had a brown dorsum with darker brown markings,
a white spot below the eye, and a narrow white labial stripe. The belly was
white; the flanks were brown with white spots, and the posterior surfaces of
the thighs were yellowish tan. The iris was a golden bronze color with much
black reticulation.
Remarks.—This species has been confused with Smilisca sordida; most
authors have referred both species to Hyla (Smilisca) gabbi. Examination of
the types of Hyla sordida, gabbi, salvini, and nigripes revealed that all of the
names were referable to a single species (S. sordida), and that the small, blunt-snouted
species in Panamá and southern Costa Rica probably was without a
name. Possibly Hyla molitor Schmidt (1857) is based on the species that
we have named S. sila, but several discrepancies in his description, plus the
unknown provenance of the type, have led us to discount the applicability
of that name to the species under consideration.
Distribution.—Smilisca sila ranges along the Pacific slopes and lowlands of
southern Costa Rica and Panamá at elevations from sea level to about 1300
meters; in northern South America the species occurs in the Caribbean lowlands
and in the valleys of the northward draining rivers of Colombia (Fig. 3).
Specimens examined, 234, as follows: Costa Rica: Puntarenas: 6 km. E
Golfito, KU 91717; Quebrada Boruca, 22 km. E Palmar Norte, KU 64265-6;
Río Zapote, 7 km. E Palmar Norte, USC 7100 (2). San José: San Isidro el
General, KU 28200; 14 km. NW San Isidro el General, USC 7098 (2); 15 km.
WSW San Isidro el General, USC 7097.
Panama: Canal Zone: Barro Colorado Island, AMNH 62320-3, CNHM
13324, 13326-8, 13330, 13338, 13359, 13423-5, KU 80460-6, 80619 (young),
80625 (skeleton), UMMZ 63542-6, USC 7051. Chiriquí: Boquete, AMNH
69815, UMMZ 58441-5; El Volcán, KU 77413, 91828-31 (skeletons), 91852-74,
91832 (eggs), 91833 (tadpoles); 6 km. S El Volcán, CNHM 60442; 16 km.
NNW El Volcán, KU 91879-90; Finca Palosanto, 6 km. WNW El Volcán,
[Pg 323]
KU 77406-12, 77692 (skeleton), 91875-7, 92330-1; Río Colorado, 17 km.
NNW El Volcán, KU 91878, 92332; Valle Hornito, 19 km. NE Gualaca, KU
92333-45. Coclé: El Valle, AMNH 55440-5 (13), 59607-14, CNHM 48140,
60349-2, 60387-92, 60401-4, 60443, 67842-5, KU 91834 (young), 91902-4,
TNHC 23751-2, USNM 140653. Colón: Río Candelaria, AMNH 53708-15,
CNHM 67826-36. Darién: Camp Creek, Camp Townsend, AMNH 40756-7,
40936-9, 40992; Río Chico, AMNH 39784, 40986-7; Río Pita, CNHM 67823-5;
Tacarcuna, USNM 141796-802; Three Falls Creek, AMNH 41684, 51788.
Los Santos: Cerro Hoya, USNM 148213-4; Lajamina, Río Puria, KU 67915.
Panamá: Altos de Pacora, KU 91894; Cerro Jefe, KU 91895-6; Cerro La
Campana, CNHM 67846, KU 91897-900, USNM 139689; Finca La Sumbadora,
KU 80467-81, 80620 (tadpoles), 91910 (eggs), 91911-2 (tadpoles), 91913
(young), 91908-9 (skeletons); Río Calobra, USNM 53722, Río Pacora, 9 km.
NNE Pacora, KU 91901. Veraguas: Cerro Carbunco, USNM 129066; Cerro
Tute, CNHM 67837-41; Isla Cebaco, Río Platanal, KU 91891-3.
Colombia: Antioquia: Urabá, Villa Arteaga, CNHM 63893 (Goin). Atlantico:
Sabanalarga, Río Causa, AMNH 14506.
Smilisca sordida (Peters), new combination
[Syntypes.—ZMB 3141 (two specimens) from "Veragua," Panamá; J.
von Warszewicz collector]. Brocchi, Mission scientifique au Mexique
..., pt. 3, sec. 2, Études sur les batrachiens, p. 42, 1881. Boulenger,
Catalogue Batrachia Salientia in British Museum, p. 393, Feb. 1, 1882.
Günther, Biologia Centrali-Americana: Reptilia and Batrachia, p. 273,
Sept. 1901. Nieden, Das Tierreich, Amphibia, Anura, I, p. 258, June,
1923.
1876 [Syntypes.—USNM 30658-9 from near Sipurio, Limón, Costa Rica;
William M. Gabb collector]. Brocchi, Mission scientifique au Mexique
..., pt. 3, sec. 2, Études sur les batrachiens, p. 37, 1881. Boulenger,
Catalogue Batrachia Salientia in British Museum, p. 372, Feb. 1, 1882.
Cope, Bull. U. S. Natl. Mus., 32:32, 1887. Günther, Biologia Centrali-Americana:
Reptilia and Batrachia, p. 274, Sept. 1901. Werner, Abhand.
Konigl. Akad. Wissen. München., 22:351, 1903. Nieden, Das Tierreich,
Amphibia, Anura I, p. 252, June, 1923. Taylor, Univ. Kansas Sci. Bull.,
35(1):840, July 1, 1952. Cochran, Bull. U. S. Natl. Mus., 220:54, 1961.
1876 [Syntypes.—USNM 30685-6, from Pico Blanco, Costa Rica; William
M. Gabb collector]. Brocchi, Mission scientifique au Mexique ...,
pt. 3, sec. 2, Études sur les Batrachiens, p. 38, 1881. Boulenger, Catalogue
Batrachia Salientia in British Museum, p. 394, Feb. 1, 1882. Cope,
Bull. U. S. Natl. Mus., 32:32, 1887. Günther, Biologia Centrali-Americana:
Reptilia and Batrachia, p. 278, Sept., 1901. Nieden, Das Tierreich,
Amphibia, Anura I, p. 253, June, 1923. James, Copeia, 3:147,
Sept. 30, 1944. Taylor, Univ. Kansas Sci. Bull, 35(1):853, July 1, 1952.
Cochran, Bull. U. S. Natl. Mus., 220:56, 1961.
p. 372, Feb. 1, 1882 [Syntypes.—BMNH 1947.2.24.13-14 from Cartago,
Costa Rica; Osbert Salvin collector]. Günther, Biologia Centrali-Americana:
Reptilia and Batrachia, pl. 71, Fig. B., Sept., 1901. Werner,
Abhand. Zool.-Bot. Gesell. Wien, 46:8, Sept. 30, 1896.
4:303, Dec. 30, 1960.
Diagnosis.—Size moderate ([M] 45 mm., [F] 64 mm.); skull slightly wider than
long, having large and elongate frontoparietal fontanelle; supraorbital flanges
absent; squamosal small, not contacting maxillary; bony section of ethmoid
terminating just anterior to anterior edge of orbit; tarsal fold weak, full length
of tarsus; inner metatarsal tubercle long, low, flat, elliptical; lips thin and flaring;
[Pg 324]
fingers one-half webbed; toes four-fifths webbed; diameter of tympanum
about one-half that of eye; no white labial stripe; dorsal dark markings irregular,
sometimes forming broad transverse bars; pale flecks on flanks and
usually on posterior surfaces of thighs; vocal sacs in breeding males white.
(Foregoing combination of characters distinguishing S. sordida from any other
species in genus.)
Description and variation.—Ten breeding males from 15 to 20 kilometers
west-southwest of San Isidro el General, San José, Costa Rica, have snout-vent
lengths of 38.1 to 42.6 mm. (40.5 mm.). In these specimens, the tibia/snout-vent
length ratio is 0.50 to 0.54 (0.52), and the tympanum/eye ratio is 0.45
to 0.57 (0.49). Specimens from the Pacific slopes of Costa Rica are larger
than those from the Meseta Central and the Caribbean lowlands. Ten males
from 6 kilometers east of Golfito, Puntarenas, have snout-vent lengths of 38.4
to 44.6 mm. (41.8 mm.), and five males from Rincón, Peninsula de Osa, have
snout-vent lengths of 38.8 to 41.6 mm. (40.3 mm.). Snout-vent lengths of
ten males from La Fortuna, Alajuela, are 31.9 to 36.0 mm. (34.4 mm.), of
ten males from Pandora, Limón, 33.8 to 37.6 mm. (35.9 mm.), and of ten
males from Escazú and Río Jorco on the Meseta Central, 34.3 to 37.6 mm.
(36.0 mm.). Eight females from the Río Jorco on the Meseta Central have
snout-vent lengths of 48.8 to 53.8 mm. (50.4 mm.), and six females from
various localities on the Pacific slopes of Costa Rica have snout-vent lengths of
56.5 to 64.0 mm. (59.8 mm.). The only noticeable differences in proportions
between males and females is in the tympanum/eye ratio; for example, this
ratio is 0.47 to 0.53 (0.49) and 0.54 to 0.68 (0.61) in ten males and eight
females, respectively, from the Meseta Central.
The shape of the snout and the associated cranial elements of S. sordida
vary geographically and ontogenetically. Specimens from the Caribbean lowlands
have blunt snouts in lateral view; those from the Pacific lowlands have
longer, more slender snouts that are pointed in lateral view, and those from
the Meseta Central are intermediate in snout shape between the two lowland
populations (Fig. 4). These differences in shape of the snout are dependent
on the nature of the underlying cranial bones, principally the maxillaries and
nasals. In specimens from the Caribbean lowlands the nasals are long, wide,
and narrowly separated from the ethmoid; the anterior edge is just posterior to
the nostril. The maxillary flanges are nearly vertical. In specimens from the
Pacific lowlands the nasals are relatively shorter, narrower, and rather widely
separated from the ethmoid; the anterior edges of the nasals do not extend so
far forward as in specimens from the Caribbean lowlands. The maxillary
flanges slant medially. In these cranial characters, specimens from the Meseta
Central are intermediate between the two lowland populations.
Superimposed on this geographic variation are ontogenetic changes, which
are most noticeable in males. In smaller, and presumably younger, specimens
the snouts are more pointed than in larger specimens; consequently some small
males from the Caribbean lowlands resemble larger males from the Pacific
lowlands, since the nasals and maxillaries of the former are not fully ossified.
In addition, in small breeding males the ethmoid is only about one-half ossified,
a large frontoparietal foramen is present, the anterior arm of the squamosal
extends only about one-fourth the distance to the maxillary (two-thirds the
distance in larger specimens), and the tegmen tympani are short, as compared
with the long, thin elements in larger specimens.
[Pg 325]
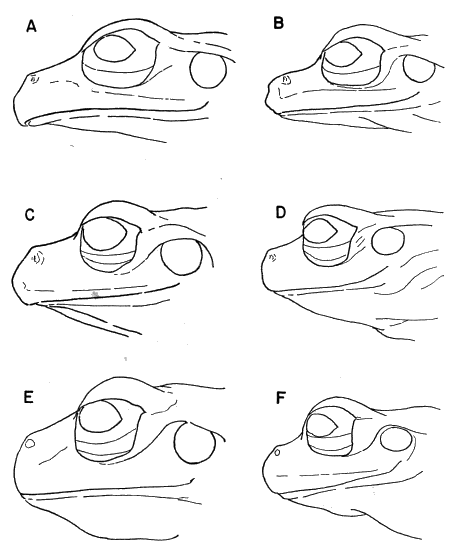
females, right column males; all from Costa Rica: (A) Camp Seattle, Rincón
de Osa, Puntarenas Prov. (UMMZ 123684); (B) Quebrada Agua Buena, 3 km.
SW Rincón de Osa, Puntarenas Prov. (USC 7236); (C) Río Oro, 28.5 km. NW
Villa Neily, Puntarenas Prov. (KU 91742); (D) Río Jorco, near Desamparados,
San José Prov. (KU 91765); (E-F) Bambú, Limón Prov. (USC 7183). ×3.
The dorsal ground-color of Smilisca sordida is gray to pale tan or reddish
brown; the venter is white. The dorsum is variously marked with dark gray,
dark brown, reddish brown, or olive-green spots or blotches (Pl. 7C). A dark
interorbital bar usually is present. The dorsal markings on the body usually
consist of a blotch, or two or more spots, on the occiput, in the scapular region,
and in the sacral region. In many specimens, especially females, these markings
are in the form of broad transverse bars. A female (USC 7164) from
[Pg 326]
Las Cañas, Guanacaste, Costa Rica, has a tan dorsum with many black flecks
and round brown spots bordered by darker brown. One female (KU 91763)
from the Río Jorco, San José, Costa Rica, has a unicolor tan dorsum. Some
individuals have scattered, small white spots on the dorsum; these are most
evident in a male (USC 7153) from La Fortuna, Alajuela. White labial stripes
and anal stripes are absent in all specimens.
The limbs are marked by dark brown transverse bars; these are indistinct
in some specimens from the Meseta Central and Caribbean lowlands, whereas
they are distinct in all specimens from the Pacific lowlands. Specimens from
the Caribbean lowlands have two to six bars on each shank, whereas specimens
from the Pacific slopes have four to six bars on each shank, and specimens
from the Meseta Central have as many as eight bars on each shank. A
narrow, sometimes broken white line is present on the ventrolateral edge of
the forearm. The webbing on the hand is tan or pale gray, and the ventral
surfaces of the tarsi and the webbing on the feet are dark gray or brown.
Breeding males have dark brown nuptial excrescences on the prepollex.
The flanks and posterior surfaces of the thighs usually are marked by bluish
white and creamy tan flecks, respectively, but vary considerably. In specimens
from the Caribbean lowlands a small amount of flecking is present in the
inguinal region, and on the posterior surfaces of the thighs flecks are few or
absent. In specimens from the Meseta Central, numerous large flecks or
small, round spots (pale bluish white in life) are on the posterior half of the
flanks; small flecks are on the posterior surfaces of the thighs. Specimens
from the Pacific slopes and lowlands of southern Costa Rica (Puntarenas and
San José Provinces) have bold mottling of black and bluish white on the
flanks and many bluish white flecks on the posterior surfaces of the thighs.
The flanks are reticulated from the axilla to the groin in two females (UMMZ
123684 and USC 7236) from Rincón, Peninsula de Osa. In specimens from
the Pacific slopes of Guanacaste in northwestern Costa Rica, flecks are present
in the inguinal region; indistinct flecks are on the posterior surfaces of the
thighs.
The throat is immaculate in specimens from the Caribbean lowlands in
Limón Province; the throats are dusky laterally in most other specimens except
some from the Meseta Central, in which the throats are heavily flecked with
black. This variation occurs in males and females.
The color and pattern in life are highly variable. A composite description
of living individuals (now KU 91718-41) from 6 kilometers east of Golfito,
Puntarenas, Costa Rica, illustrates the variability: "Dorsum pale olive-green,
fading to tan posteriorly, or tan all over with dark olive-green or dark brown
spots on back and bars on limbs. Flanks dark brown with cream, greenish
gray, or bluish gray mottling. Posterior surfaces of thighs dark brown with
pale blue, pale green, or tan flecks. Iris creamy silver. Throats white with
some brown flecks peripherally." (Duellman, Field notes, February 15, 1965.)
A male from the Río Jorco, San José, Costa Rica, was dull olive-tan above
with olive-green marks; the flanks were brown with pale tan flecks, and the
posterior surfaces of the thighs were pale brown with cream-colored flecks.
Six females from the same locality were reddish brown above with olive-brown
or dark brown markings; one was uniform orange-tan, and another was dull
olive-green with darker markings.
The color of the iris in living frogs varies from creamy silver to grayish
yellow or bronze with a variable amount of black reticulation.
[Pg 327]
Natural History.—Smilisca sordida is not associated with any one type of
vegetation; instead it lives in the vicinity of rocky streams having low gradients.
Breeding takes place primarily in the dry season, when the water in the
streams is clear and at a low level. Through most of the range of S. sordida
showers, or even short heavy rains, occur in the dry season. After such rains
the breeding activity is maximal. Breeding congregations have been found
from December through April, but a few calling males and gravid females
have been taken in June, July, and August. In the rainy season non-breeding
individuals are found sitting on bushes near streams at night. Taylor (1952:843)
found specimens in bromeliads by day.
Males usually call from rocks or gravel bars in, or at the edge of, streams.
Some individuals perch in low bushes overhanging the streams, and some sit
in shallows in the streams. Clasping pairs have been found on the banks of
streams and in shallow water in streams.
The breeding call consists of one to six moderately short, rather high-pitched
notes (duration 0.18 to 0.45 seconds) repeated at intervals of 12 seconds to
several minutes. Each note is a vibrant rattle having 78 to 135 pulses per
second and major frequences of about 1200 to 2600 cycles per second (Pl. 11C).
The tadpoles live in shallow parts of the streams, where they cling to the
surfaces of small rocks and hide beneath leaves and rocks. A complete developmental
series of tadpoles is not available; measurements of those stages
examined are summarized in Table 12.
A typical tadpole in stage 36 of development (KU 68475 from 15 km.
WSW of San Isidro el General, Costa Rica) has a body length of 11.7 mm.,
tail length of 22.8 mm., and a total length of 34.5 mm.; body about three-fourths
as deep as wide; snout broadly rounded in dorsal view, sloping and
rounded in lateral view; eyes widely separated, directed dorsolaterally; nostril
slightly closer to eye than to tip of snout; mouth ventral; spiracle sinistral,
about two-thirds distance from snout to posterior end of body and slightly
below midline; anal tube dextral; caudal musculature heavy, straight; dorsal
fin not extending onto body; fins deepest at about mid-length of tail; there
depth of caudal musculature equal to depth of dorsal fin and half again as
deep as ventral fin; musculature extending nearly to tip of tail; body reddish
brown above and pale grayish brown with white flecks below; caudal musculature
pale tan with brown flecks; a series of reddish brown dashes at base of
caudal fin separated from others in series and from dashes on other side by
creamy white; fins transparent with reddish brown flecks on posterior one-half
of ventral fin and on all of dorsal fin (Fig. 14C). Mouth bordered by
two rows of short, pointed papillae; lateral fold present; tooth-rows 2/3; upper
rows equal in length; second upper row narrowly interrupted medially; three
lower rows complete, nearly as long as upper rows, deeply indented medially;
upper beak robust, inner surface not forming continuous arch with short lateral
processes; lower beak deep, V-shaped; both beaks bearing short serrations
(Fig. 15F).
Little variation occurs in structure. In some specimens the second upper
tooth-row is complete; no individuals were found to have the row broadly
interrupted medially.
The series of dark dashes on the dorsal edge of the caudal musculature is
diagnostic of all stages studied. In life, tadpoles from 15 and 20 kilometers
west-southwest of San Isidro el General, Costa Rica, had a tan body, often
[Pg 328]
with an olive-tan tinge; the caudal musculature was tan; the flecks and dashes
were dull red or reddish brown. Tadpoles from 6 kilometers east of Golfito,
Costa Rica, had bodies with olive-green flecks. The caudal musculature was
brown with bluish green flecks; the fins were transparent with reddish brown
flecks. The belly was a silvery golden color. Tadpoles from Bajos de Jorco,
Costa Rica, had brown bodies with bluish green flecks; the tail and fins had
reddish brown flecks and dashes. The iris was a bronze color in specimens
from all three localities, as well as in the young mentioned in the following
paragraph.
Nine recently metamorphosed young were found on vegetation at the edges
of streams in April. These specimens have snout-vent lengths of 13.1 to
15.7 mm. (14.9 mm.) and in life were pale greenish tan or olive-tan above
and white below. The hands, feet, and thighs were pale yellowish tan.
Remarks.—The foregoing synonymies indicate that confusion has existed in
the application of various names, to this species, as well as in use of the names
sordida and gabbi to include the species that we describe and name Smilisca
sila. Correct allocation of the names involved was possible only after studying
and comparing the type specimens, for the descriptions given by the various
authors are not sufficiently explicit to determine the nature of many essential
features.
The presence of a rounded snout and a long white throat in males distinguishes
S. sordida from S. sila, which has a high truncate snout and short dark
throat in males. The two syntypes of Hyla sordida Peters, 1863, (ZMB 3141)
are males having snout-vent lengths of 36.9 and 37.0 mm. The two syntypes
of Hyla gabbi Cope, 1876 (USNM 30658-9), are females having snout-vent
lengths of 52.8 and 53.7 mm., respectively. Also included in the collections
made by Gabb is eastern Costa Rica are two males (USNM 30685-6), which
Cope (1876) named and described as Hyla nigripes. These specimens are
soft and faded, but are recognizable as the same as Hyla sordida Peters; the
syntypes of Hyla nigripes have snout-vent lengths of 37.6 and 37.7 mm. We
have examined one of the syntypes of Hyla salvini Boulenger, 1882 (BMNH
1947.2.24.13), a female having a snout-vent length of 54.6 mm. We are
convinced that all of these type specimens are representatives of one species,
the earliest name for which is Hyla sordida Peters, 1863. The type localities
for three of the named species are in Costa Rica—H. gabbi from Sipurio on
the Caribbean lowlands, H. nigripes from the Caribbean slopes of Pico Blanco,
and H. salvini from Cartago on the Meseta Central. The type locality of H.
sordida was given as "Veraguas" by Peters (1863). At that time Veraguas
was often considered to be most of western Panamá. Though we have not
seen Panamanian specimens other than the types of S. sordida and one specimen
from the Pacific lowlands of western Panamá, the species probably occurs
on the Caribbean slopes of western Panamá. The species has been taken on
the Caribbean lowlands of Costa Rica within a few kilometers of Panamá;
collecting on the Caribbean slopes in the provinces of Bocas del Toro and
Veraguas should reveal the presence of Smilisca sordida there.
Distribution.—Smilisca sordida is found along the Pacific slopes and lowlands
from Guanacaste, Costa Rica, southeastward to extreme western Panamá,
to elevations of about 1200 meters on the Meseta Central in Costa Rica, and
on the Caribbean slopes and lowlands of Costa Rica and probably adjacent
Panamá (Fig. 5). One specimen purportedly comes from "Río Grande,
Nicaragua."
[Pg 329]
showing locality records for Smilisca sordida.
Specimens examined.—412, as follows: Nicaragua: "Río Grande" (?
Depto. Zelaya), MCZ 2634.
Costa Rica: Alajuela: Between Atena and Salto de San Mateo, USC
6185; 8 km. N Ciudad Quesada, USC 7155 (4); La Fortuna, USC 7153 (20);
3 km. E La Fortuna, USC 7150; San Carlos, USNM 29969; Sarchi, KU 32990-9,
36792-3.
Cartago: Cartago, BMNH 1947.2.24.13; headwaters of Río Pacuare, USC
119; Instituto Interamericano de Ciéncias Agricolas, Turrialba, KU 37012,
USC 420, 437; Río Reventazón, Turrialba, MCZ 29268: 10 km. N Río
Reventazón bridge, USC 7073; 5 km. SW Río Reventazón bridge on Paraiso-Orosi
road, USC 669; Turrialba, UMMZ 118405, USC 455, USNM 29936-9.
Heredia: Puerto Viejo, KU 36791.
Guanacaste: Las Cañas, USC 7164; Santa Cecilia, MCZ 7924-5; Tilarán,
USC 7161 (5).
Limón: Bambú, USC 7171 (2), 7183 (13); La Lola, USC 820 (6), 6083-94,
8064, 8071; Pandora, USC 7188 (7), 7189, 7190 (3), 7191 (5); Pico Blanco,
USNM 30685-6; Río Larí, 14-16 km. SW Amubre, USC 7179, 7180 (10);
Sipurio, USNM 30658-9; Suretka, KU 36764, 36765 (skeleton), 36766-78.
Puntarenas: 6 km. N Dominical, KU 91749-50, 91811 (young), 91812 (tadpoles);
Esparta, MCZ 8028; 6 km. E Golfito, KU 91718-41, 91809 (young),
91810 (tadpoles), 91816-9 (skeletons), USC 7103 (23); Quebrada Agua Buena,
3 km. SW Rincón de Osa, USC 7236 (6); Quebrada Boruca, 22 km. E Palmar
Norte, KU 64264; Rincón de Osa, Camp Seattle, UMMZ 123680-5, S-2792
[Pg 330]
(skeleton), USC 705 (5), 6023, 7254; Río Barranca, USC 7119 (2); Río Ceiba,
6 km. NW Buenos Aires, KU 91747-8, USC 7112 (7); Río Ciruelitas, 16 km.
NW Esparta, USC 7121 (3); Río Claro, 14.2 km. NW Villa Neily, USC 7110
(4); Río Ferruviosa, 7 km. S Rincón de Osa, USC 7235 (4); Río Lagarto at
Pan-American Hwy. (Guanacaste Border), USC 7122 (4); Río La Vieja, 30 km.
E Palmar Norte, KU 87684 (tadpoles), 91743-6, USC 7083 (2); Río Oro, 28.5
km. NW Villa Neily, KU 91742; Río Volcán, 10 km. W Buenos Aires, USC
7113; Río Zapote, 7 km. E Palmar, USC 7100 (4); 3-5 km. W Palmar, USC 7101
(18); 7 km. SE Palmar, KU 64261-3; 1.2 km. NW Villa Neily, USC 8032;
3 km. NW Villa Neily, USC 7109 (20); 5 km. NW Villa Neily, USC 6176,
8035.
San José: Bajos de Jorco, KU 91813 (tadpoles); Escazú, KU 34863, 34869-75,
USC 813; between Monrovia and La Hondura, ± 0.5 km. N Santa Rosa,
USC 302 (2); Paso Ancho, Río Jorco, UMMZ 122649 (6), USC 530 (3); Río
Jorco, near Desamparados, KU 91757-65, 91796-7, 91820-3 (skeletons), USC
228, 513, 7117 (7); Río Peje, 10 km. SSE San Isidro el General, USC 7115
(3); Río Tiriví, MCZ 7972; San Isidro el General, CNHM 101096, KU 28201,
32989, UMMZ 72024; 15 km. WSW San Isidro el General, KU 64245-56, 68473
(tadpoles), 68474 (young), 68475 (tadpoles), 86516, 91754-6, 91793-5, USC
7097 (6); 17.1 km. WSW San Isidro el General, USC 6047; 18 km. WSW San
Isidro el General, USC 689; 20 km. WSW San Isidro el General, KU 64257-9,
64260 (skeleton), 68468 (young), 68469 (tadpoles), 68470 (young), 68471-2
(tadpoles), 68476 (young), 68633-4 (skeletons), 91751-3; San José, AMNH
7501-4, USC 298; Santa Rosa, Río Virilla, USC 7145.
Panama: Chiriquí: Río Jacu, 5.8 km. ESE Paso Canoas, KU 91905.
"Veraguas," ZMB 3141 (2).
Osteology
In attempting to assay the taxonomic significance of skeletal differences
we are faced with a dearth of data on the skeletons of frogs in general and
hylids in particular. Recent reviews by Brattstrom (1957) and Hecht (1962,
1963) have been concerned with general salientian classification and phylogeny,
principally at the family level. Savage and Carvalho (1953), Griffiths (1959),
and Baldauf (1959) used osteological characters in determining the taxonomic
status of the families Pseudidae, Brachycephalidae, and Bufonidae, respectively.
Carvalho (1954) presented osteological evidence for the generic
separation of New World microhylids. Zweifel (1956) and Tihen (1962)
used osteological characters at the levels of the species-group and species in
their respective studies on Scaphiopus and Bufo. Little has been recorded
about the skeletons of the hylids. Goin (1961) mentioned dentigerous elements
and cranial co-ossification in his synopsis of the genera of hylids.
Copland (1957) in his review of the Hyla of Australia, Funkhouser (1957)
in her revision of Phyllomedusa, and Zweifel (1958) in his review of Nyctimystes
did not consider skeletal characters.
Some osteological studies on hylids have yielded worthwhile information.
Mittleman and List (1953) used osteological characters in defining the genus
Limnaoedus: Starrett (1960) used cranial characters in combination with jaw
musculature in defining the genus Smilisca, and Duellman (1964) used cranial
characters in delimiting the Hyla bistincta group. Brief descriptions of cranial
structure were given for Phrynohyas (Duellman, 1956) and Ptychohyla
(Duellman, 1963a); specific and sexual differences in the skulls of Hyla
chaneque and Hyla taeniopus were pointed out by Duellman (1965). Stokely
[Pg 331]
and List (1954) described early cranial development in the hylid Pseudacris
triseriata triseriata.
Because our knowledge of the skeleton in hylids is so incomplete, we are
not attempting to place Smilisca in the general scheme of hylid phylogeny on
the basis of skeletal characters. Instead, our purposes are to describe the
skeleton and its ontogenetic development in one member of the genus (S.
baudini), and to make comparisons that show taxonomic differences in osteological
characters among species of Smilisca.
The study of 68 dried skeletons and 25 cleared and stained preparations,
including an ontogenetic series of S. baudini, has resulted in an understanding
of the progressive development of skeletal elements and a knowledge of interspecific
and intraspecific variation in these elements. Furthermore, investigations
of the osteology have provided correlations between some cranial characters
and certain aspects of external morphology.
Descriptive Osteology of Smilisca baudini
The following description is based primarily on an adult female (KU
68184):
Skull.—The skull is large, solid, and broader than long; the greatest width
is between the sutures of quadratojugal and maxillary on either side of the
skull (Pls. 2-3). The maxillaries bear well-developed dorsal flanges, curve
gently, join the moderately convex premaxillaries anteriorly and form a slightly
truncate snout. The combined premaxillary width is about one-fourth the
width of the skull. The premaxillaries are separated medially, and laterally
from the maxillaries by sutures. Each premaxillary bears a dorsomedial alary
process, which is anteriorly convex and four times as high as the depth of the
lateral wing of premaxillary; each premaxillary also has a ventromedial palatine
process that projects dorsally from the lingual edge of the premaxillary. The
septomaxillaries are closely associated dorsally with the premaxillaries immediately
lateral to the prenasal processes.
The nasals are large, widest anteriorly and narrowing posteriorly, parallel to
maxillaries, and not separated from the ethmoid by cartilage. The nasals bear
long, delicate maxillary processes extending nearly to the maxillaries. Anteriorly,
the nasals are widely separated by the partially ossified internasal septum,
which is in contact with the premaxillaries between the prenasal processes; the
anterior points of the nasals lie approximately one-half the distance between
the anterior ends of the ethmoid and the premaxillaries. The ethmoid is large
and completely ossified; the margins are smooth. The trunate anterior edge
lies between the nasals and is in contact with the internasal septum. The
frontoparietals are large, smooth-margined, and bear large supraorbital flanges
curving posterolaterally at the rear of the orbit. A small, oval foramen involves
the posterior part of the ethmoid and anterior portion of frontoparietals;
continued ossification in older specimens fills in the foramen, thereby resulting
in a solidly roofed cranium. The auditory regions are relatively massive and
bear narrow tegmen tympani; the distal ends of the tegmen tympani are
medial to the lateral edge of the pterygoids in dorsal view. The squamosals
are large; the long anterior arm is separated from the maxillary by a suture.
The delicate, spindle-shaped columellae lie ventral to the tegmen tympani and
squamosals, are spatulate distally, and have a broad basal attachment to the
auditory region.
The vomers are moderately large and are in contact anteriorly with the
premaxillaries and posteriorly with the ethmoid. Each vomer has two wide
serrated flanges laterally. The tooth-bearing parts of the vomers are widely
separated and at a slight angle to one another; the vomers terminate medially
in two pointed processes on the ethmoid. The palatines are edentate, but
bear strong ridges throughout their lengths. They are broadly in contact with
the maxillary, are narrow medially, and are attached by pointed processes to
[Pg 332]
the medial part of the ethmoid. The pterygoids are large, attached to the
maxillaries immediately anterior and medial to the squamosal-maxillary connection,
bear well-developed pedicles, which are broadly attached to the
proötic, and a wide wing is in contact posteriorly with the distal two-thirds of
the quadrate.
The angular makes up most of the lower jaw, bears a broad articular surface
posteriorly, and has a small coronoid process on the lingual edge; anteriorly
the angular is separated from the dentary and mentomecklian by Meckel's
cartilage. The dentary lies external to the angular and extends from the
mentomecklian to approximately the mid-length of the angular. The mentomecklians
are ossified, but separated by cartilage medially.
Hyoid.—The hyoid plate is curved, thin, and mostly cartilaginous, but
calcined posteriorly (Fig. 6). The anterior cornua are slender, cartilaginous,
and curve anteromedially from the hyoid plate and thence laterally and
posteriorly, to attach to the posterior surface of the proötics. The lateral
cornua are broad, flat, cartilaginous lateral extensions from the bases of the
anterior cornua. The posterior cornua are bony, except distally.
baudini showing areas of muscle attachment: Gen. L., attachment of
geniohyoideus lateralis; Gen. M., attachment of geniohyoideus medialis;
Hyo., attachment of hyoglossus; Omo., attachment of omohyoideus;
Pet., petrohyoideus; St., attachment of sternohyoideus. KU 64220, ×5.
[Pg 333]
Vertebral Column.—The atlas lacks transverse processes and a neural crest,
whereas transverse processes are present on the other seven presacral vertebrae,
and knoblike neural crests are present on the second, third, and fourth vertebrae;
a faint neural ridge is visible on the fifth vertebra. The transverse
processes are directed laterally on the second and sixth vertebrae, ventrolaterally
on the third, posterolaterally on the fourth and fifth, and anterolaterally on
the seventh and eighth. The processes are slightly expanded on the fourth,
and more so on the fifth, vertebra. The sacral diapophyses are expanded and
have a border of calcified cartilage laterally. There are two sacral condyles.
The slender coccyx has a thin dorsal ridge on the anterior three-fourths of its
length.
Pectoral Girdle.—The omosternum is large, ovoid, and cartilaginous; the
sternum is a thin cartilaginous sheet deeply notched posteriorly and is not differentiated
into episternal and xiphisternal elements. The coracoids are robust,
twice as stout as the clavicles. The epicoracoidal cartilages overlap in the
usual arciferal manner, except that they are fused anteriorly between the slender
clavicles. The clavicles are strongly arched. The clavicle, coracoid, and
scapula on each side form a bony articulation at the glenoid fossa. A bifurcation
of the ventral end of the scapula results in a large glenoid foramen. The
scapula is flat and expanded dorsally; the suprascapula is broad, flat, and
calcified in large adults. In young specimens no distinct ossification of the
cleithrum or ossification of endochondral centers are evident.
Arm and Hand.—The humerus is equally well-developed in both sexes and
has a prominent lateral crest. The radius and ulna are completely fused. A
bony prepollex is present in both sexes. The metacarpals are about equal in
length. The phalangeal formula is 2-2-3-3; the terminal phalanges are claw-shaped.
Pelvic Girdle.—The ilia are long, slender, and slightly curved. A thin ridge
projects laterally from the dorsal edge of the posterior one-half of each ilium.
The ilial prominence is large and knoblike when viewed from above. The
anterior edge of the ilial prominence is at the level of the anterior edge of the
acetabular border. The dorsal acetabular expansion is small. The pubis is
slender, and the ischium is elevated and robust.
Leg and Foot.—The slightly curved femur has a distinct crest proximally
on the posterior surface. The nearly straight tibio-fibula is slightly longer
than the femur. The tibial and fibial elements are completely fused but have
a distinct cleft between them. A small foramen exists at the mid-length of
the tibio-fibula. The fibulare (calcaneum) is much more robust than the
tibiale (astragalus). The prehallux is large and flat. The metatarsals of the
third, fourth, and fifth digits are equal in length; the metatarsal of the second
is somewhat shorter, and that of the first is much shorter. The phalangeal
formula is 2-2-3-4-3; the terminal phalanges are claw-shaped.
Developmental Cranial Morphology of Smilisca baudini
The following description of development of the skull of Smilisca baudini
is based on the examination of 12 cleared and stained specimens. In table 3
the cranial bones are listed in the left hand column in the approximate order
of their appearance in the young frogs. Across the top of the table selected
specimens designated by developmental stage or snout-vent length are listed.
It should be noted that although each individual, from left to right, has an
increasing number of ossified bones, the correlation with increasing size is
imperfect; the precise ages of the individuals are unknown.
The first bones to appear are the septomaxillaries, frontoparietals, part of
the exoccipital, and the parasphenoid in developmental stage 40. The frontoparietals
are represented by two slender ossifications dorsomedial to the orbits;
the septomaxillaries are present as small ossifications anterior to the nasal
capsules (Pl. 1A). The parasphenoid is present as a faint median ossification,
and the exoccipital shows some ossification.
[Pg 334]
| Table 3.—The Order of Occurrence of Cranial Ossifications in the Skull of Smilisca baudini. Where Numbers Are Divided by a Slash Mark, the Left and Right Symbols Correspond to the Left and Right Sides of the Skull, Respectively. | |||||||
| Bone | Stage 40 | Stage 44 | 12.6 mm. | 13.9 mm. | 32.0 mm. | 27.0 mm. | 20.1 mm. |
| Frontoparietal | X | X | X | X | X | X | X |
| Parasphenoid | X | X | X | X | X | X | X |
| Septomaxillaries | X | X | X | X | X | X | X |
| Exoccipitals | X | X | X | X | X | X | X |
| Squamosals | — | X | X | X | X | X | X |
| Premaxillaries | — | X | X | X | X | X | X |
| Maxillaries | — | X | X | X | X | X | X |
| Nasals | — | — | X | X | X | X | X |
| Pterygoids | — | — | X | X | X | X | X |
| Vomers | — | — | — | X | X | X | X |
| Palatines | — | — | — | X | X | X | X |
| Quadratojugals | — | — | — | X | X | X | X |
| Ethmoid | — | — | — | — | X | X | X |
| Columellas | — | — | — | — | X | X | X |
| Supraorbital Flanges | — | — | — | — | — | X | X |
| Proötics | — | — | — | — | — | — | X |
| Vomerine Teeth | — | — | 1/1 | 4/3 | 5/5 | 3/3 | 5/4 |
| Maxillary Teeth | — | 0/7 | 3/5 | 6/5 | 30/31 | 30/26 | 37/36 |
| Premaxillary Teeth | — | 2/4 | 3/3 | 5/5 | 7/6 | 8/6 | 8/7 |
The dentigerous bones are among the most rapidly developed, although
not the first to appear. They are present in developmental stage 44 before
metamorphosis is completed. The maxillaries bear a few teeth anteriorly and
are ossified posteriorly to a point one-third of the distance from the anterior
to the posterior edge of the orbit. Ossification lengthens the posterior termini
of the maxillaries to the posterior edge of the orbit. In front of the anterior
margin of the orbit, bone is proliferated dorsal to the main axes of the maxillaries
and forms moderate dorsal maxillary flanges. The premaxillaries appear
simultaneously with the maxillaries. Initially they are widely separated
medially from each other, and laterally from the developing maxillaries; each
bears two or three teeth, large dorsally blunt alary processes, and small
[Pg 335]
palatine processes. The median and lateral edges of the prenasal processes
lengthen heterochronously, causing the median edges to be longest and to lie
slightly dorsal to the level of the septomaxillaries. After the maxillaries and
premaxillaries develop, the vomers appear as small horizontal ossifications
anterior to the parasphenoid. Ossification begins in the lateral flanges, then
in the prevomerine processes, and lastly in the posterior dentigerous parts of
the bones; the prevomerine processes are the last parts of the vomers to ossify
completely.
Initially the frontoparietals are present as thin rods of ossification dorsomedial
to the orbits; the frontoparietals extend from the anterior to the
posterior end of the orbit by developmental stage 44. The anterior ends of
the bones remain thin and pointed; ossification progresses medially from the
midpoint of the length of the orbit and posteriorly to the level of the exoccipital;
a median center of ossification joins the frontoparietals posteriorly,
thereby forming the posterior border of the frontoparietal fontanelle. The
supraorbital flanges of the frontoparietals do not appear until all other cranial
bones are ossified, or nearly so. The most rapid ossification begins laterally
at the posterior edge of the orbit and decreases anteriorly over the posterior
half of the orbit. This differential rate of proliferation of bone results in the
pattern of development of the supraorbital flanges shown in figure 7. The
nasals appear as thin slivers of bone half way between the anterior ends of the
frontoparietals and the end of the snout. As ossification proceeds the nasals
assume a triangular shape in dorsal view. The anterior ends are pointed;
the lateral margins are parallel to the maxillaries. The posteromedial points
do not reach the lateral margins of the ethmoid, and the maxillary processes
extend about three-fourths the distance from the bodies of the nasals to the
maxillaries. Following the union of the frontoparietals posteriorly, the nasals
widen anteriorly and are narrower at the midpoints of their long axes than
anteriorly or posteriorly. With further ossification the maxillary processes
extend to the maxillaries and form complete bony anterior margins to the
orbits; the mid-parts of the nasals widen (Pl. 1B).
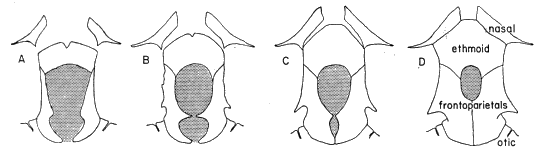
bony elements in Smilisca baudird: (A) KU 60026, ×5; (B) KU 85438,
×4; (C) KU 26328, ×3; (D) KU 68184, ×2.3.
The parasphenoid is the first of the palatal bones to appear. At metamorphosis
the bone is well developed; the anterior tip is situated just in front
of the anterior edge of the orbit, and posteriorly the lateral processes extend
laterally beyond the ossified parts of the auditory region. The pterygoids do
not appear until metamorphosis, when ossification is evident in only the mid-parts
of the posterolateral arms. Ossification follows in the mid-parts of the
[Pg 336]
anterolateral arms and occurs last in the pterygoid pedicles. The palatines do
not appear until all three arms of the pterygoids are at least partly ossified.
Ossification proceeds rapidly from the maxillaries medially to the unossified
ethmoid, which is the last of the cranial bones to appear. Initially it is extremely
shallow; dorsally it is widely separated from the nasals, and ventrally
the posterior margin meets the anterior point of the parasphenoid. In dorsal
view, ossification proceeds anteriorly between the nasals and posteriorly, ventral
to the frontoparietals; ventrally, ossification proceeds posteriorly dorsal to the
parasphenoid.
The ventral arms of the squamosal and the supraoccipital region of the
exoccipital are the first occipital bones to appear. Ossification follows in the
regions of the semicircular canals and occipital condyles. The dorsal end of
the ventral arm of the squamosal and the posterior arm of the squamosal
ossify as a unit at the same time the quadratojugal appears. Shortly thereafter
the anterior arm of the squamosal ossifies, the distal part of the columella
appears, and the anterior and lateral parts of the auditory region ossify.
The angular and dentary of the lower jaw appear concurrently with the
dentigerous bones. Initially, the angular is short and broad; the articular surface
is absent, and the anterior end is slightly overlapped by the dentary.
The mentomecklians do not ossify until approximately the same time that the
quadratojugal appears in the upper jaw.
Comparative Osteology
The genus Smilisca is characterized by the following combination of cranial
osteological characters: (1) A large amount of bone is involved in the skull
and a minimal amount of cartilage and/or secondarily ossified cartilage; co-ossification
is absent. (2) The skulls are uniformly broad with angular lateral
margins, and truncate anteriorly. (3) An internasal septum and quadratojugals
are present. (4) A well-developed squamosal minimally extends one-fourth the
distance from the dorsal end of the quadrate to the maxillary, and maximally
is separated from the maxillary by a suture. (5) The ethmoid is large; the
distance between the anterior end of the ethmoid and the anterior edge of
the premaxillary varies between 15 and 20 per cent of the total length of the
skull.
On the basis of cranial osteology two species-groups can be recognized
within the genus Smilisca. The sordida group, comprising S. sordida and puma,
is characterized by a broad skull in which the lateral margins of the maxillaries
are relatively straight anterior to the orbit. The moderate-sized nasals are
rounded anteriorly, and bear relatively short, sometimes blunt, maxillary
processes. The long axes of the nasals are not parallel to the maxillaries.
The ethmoid is proportionately small in the sordida group. The bony part of
the ethmoid terminates near the anterior edge of the orbits and does not
extend anteriorly between the nasals; the entire anterior margin of the ethmoid
is separated from the nasals by cartilage. The squamosals are generally small.
They are narrow in dorsal view, and minimally extend one-fourth the distance
from the dorsal end of the quadrate to the maxillary, and maximally, two-thirds
the distance. The tegmen tympani are relatively small (Fig. 8).
[Pg 337]
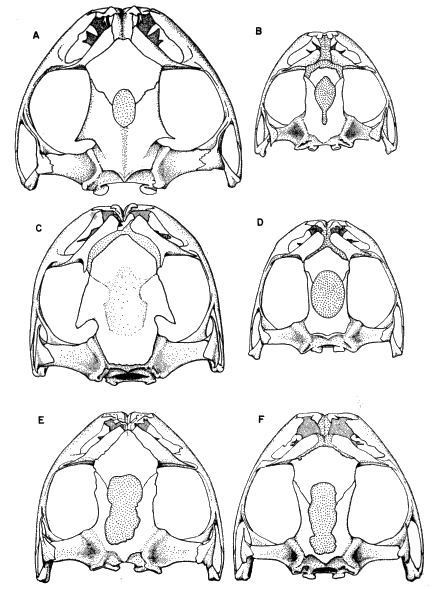
(KU 68184); (B) S. puma (KU 68636); (C) S. phaeota (KU 41090); (D)
S. sila (KU 80625); (E) S. cyanosticta (KU 55938), and (F) S. sordida (KU
36765). ×1.5.
In contrast to the tendency for reduction of cranial parts in the sordida
group, the baudini group, constituted by S. cyanosticta, phaeota, and baudini,
is characterized by more ossification of the cranial elements. The skull is
broad; the lateral margins are less angular and are gently curved, rather than
straight as in the sordida group. The nasals tend to be larger with the long
axes parallel to the maxillary. Anteriorly the nasals are pointed, and posteriorly
[Pg 338]
they bear long, delicate palatine processes extending to the maxillary. The
ethmoid is fully ossified, extends anteriorly between the nasals, and laterally
is separated by a suture from the nasals if the latter are fully ossified. The
squamosals are large, and wide in dorsal view. They minimally extend one-fourth
the distance from the dorsal end of the quadrate to the maxillary, and
maximally are sutured to the maxillary. The tegmen tympani are massive.
Smilisca sila is intermediate between the two species-groups described. The
skull is broad; the lateral margins are gently curved, and have a pronounced
angularity just anterior to the palatines which results in a broad, truncate
snout. The nasals are moderate in size; because of the anterior angularity
of the lateral margins, the long axes of the nasals lie parallel to the maxillary.
The nasals are only slightly pointed anteriorly, and posteriorly they bear short,
blunt palatine processes and medial processes in contact with the lateral
corners of the ethmoid. The ethmoid is fully ossified, but does not extend
anteriorly between the nasals. The squamosals are moderate in size and extend
one-fourth the distance from the dorsal end of the quadrate to the
maxillary. The tegmen tympani are relatively large, but proportionately short.
The cranial characters utilized in the analysis of species groups (general
shape, nature of the nasals, ethmoid, squamosals, and tegmen tympani), together
with other characters, such as the relative height and shape of the
prenasal processes, the extent of the internasal septum, and the nature of the
vomers, frontoparietals, maxillaries and pterygoids are useful in distinguishing
the various species (Table 4, Fig. 8), as well as in establishing relationships
within the species-groups.
Within the sordida group, S. sordida and S. puma can be distinguished by
the following characters: The bony part of the ethmoid terminates posterior to
the anterior edge of the orbit and is thus widely separated from the nasals by
cartilage in S. puma. In S. sordida the bony part of the ethmoid always
terminates at a level equal to, or slightly in front of the anterior edge of the
orbit; therefore, less cartilage exists between the ethmoid and nasals in S.
sordida than in S. puma. The width of the premaxillary comprises about 30
per cent of the width of the skull in S. sordida and 20 per cent in S. puma.
The proportion of the length of the skull anterior to the bony part of the
ethmoid in S. sordida is approximately 21 per cent, as compared with about 29
per cent in S. puma. The prenasal processes are convex in S. sordida and
straight in S. puma.
The marked ontogenetic variation in S. sordida is considered in more detail
in the account of that species, but it is pertinent to the present discussion to
note that with respect to some features of the skull some young breeding specimens
of S. sordida are intermediate in appearance between large females of
S. sordida and adults of S. puma. In some breeding males (usually the
smaller individuals) of S. sordida the bony part of the ethmoid terminates at
the anterior edge of the orbit and is widely separated from the nasals by
cartilage. In small individuals S. sordida, especially in males, and in adults
of S. puma the tegmen tympani are relatively short, whereas in adult females
of S. sordida these elements are long and slender. In the smaller specimens
of S. sordida and in S. puma the squamosal is small; it extends only about one-fourth
of the distance to the maxillary in the smaller S. sordida and about one-half
the distance in S. puma. The more massive squamosal in large adult
females of S. sordida extends at least two-thirds of the distance to the maxillary.
[Pg 339]
| Table 4.—Comparative Cranial Osteology of Smilisca. | ||||||
| Character | S. baudini | S. cyanosticta | S. phaeota | S. puma | S. sila | S. sordida |
| Alary Processes | Four times as high as lateral wing of premaxillary; anteriorly convex. | Three times as high as lateral wing of premaxillary; anteriorly convex. | Two and one-half times as high as lateral wing of premaxillary; anteriorly convex. | Two times as high as lateral wing of premaxillary; straight. | One and one-half times as high as lateral wing of premaxillary; straight. | Two and one-half times as high as lateral wing of premaxillary; slightly convex anteriorly. |
| Nasals | Long, wide anteriorly, narrowing posteriorly; attached to ethmoid. | Long, widest posteriorly; attached to ethmoid. | Long, widest anteriorly and posteriorly, bearing posteromedial process; not attached to ethmoid. | Short, narrow, not attached to ethmoid. | Short, wide, bearing small posteromedial processes; not attached to ethmoid. | Moderately long narrowest anteriorly and posteriorly; not attached to ethmoid. |
| Ethmoid | Long; entirely ossified; smooth margins. | Long, entirely ossified; smooth margins. | Long, entirely ossified; smooth margins. | Short, about two-thirds ossified; irregular margins. | Moderately long; entirely ossified; smooth margins | Short; one-half to entirely ossified; irregular margins. |
| Frontoparietal | Small, ovid fontanelle present or absent; long, pointed postorbital processes curving along posterior border of orbit. | Large fontanelle, two and one-half times as long as wide; narrow supraorbital flanges with irregular margins. | Fontanelle absent; large supraorbital flanges having straight edges and extending posterolaterally. | Keyhole-shaped fontanelle; smooth margins; flanges absent. | Large, ovoid fontanelle; smooth margins; flanges absent. | Large, elongate fontanelle; smooth margins; flanges absent. |
| Squamosal | Large: anterior arm in contact with maxillary. | Large; anterior arm in contact with maxillary. | Large; anterior arm extending 1/2-2/3 way to maxillary. | Small; anterior arm extending 1/2 way to maxillary. | Moderately large; anteriorarm extending 1/4 way to maxillary. | Moderately small; anterior arm extending 1/4-2/3 way to maxillary. |
[Pg 340]
Within the baudini group, the skull of S. cyanosticta is the most generalized
of the three species; the cranial characters are intermediate between S. phaeota
and S. baudini. The lateral margins of the skull in S. cyanosticta are gently
curved, and have an angularity anterior to the palatine-maxillary suture; the
anterior margins are less angular in S. phaeota, which has a broader snout.
Posteriorly in S. baudini the margins are slightly curved medially, and the
greatest width of the skull is between the quadratojugal-maxillary sutures on
either side of the skull. The frontoparietals of S. cyanosticta bear slightly
irregular lateral margins and a large fontanelle. There is a tendency for obliteration
of the fontanelle with increasing age in both S. baudini and S.
cyanosticta; the lateral margins of the frontoparietals bear large supraorbital
flanges in both of these species. In S. phaeota the flanges are most prominent;
they extend posterolaterally with straight margins along two-thirds of the
length of the orbit and terminate in rather blunt points. The broad interorbital
flanges result in a relatively broad external interorbital distance. In S. baudini
the flanges are curved posterolaterally around the orbit and terminate in sharp,
thin points. The tegmen tympani of all three species are massive. In S.
cyanosticta the proötics slope posteriorly, whereas they slope anteriorly in S.
baudini and S. phaeota.
The skulls of S. cyanosticta and S. baudini are alike in certain respects.
The squamosals of both species are large and connected to the maxillary by a
bony connection; the squamosals of S. phaeota are large, but extend only two-thirds
of the distance from the dorsal end of the quadrate to the maxillary.
In S. baudini and S. cyanosticta the nasals are separated throughout their
lengths from the ethmoid, whereas the nasals of S. phaeota are separated
from the ethmoid by cartilage. The latter separation is due to an incomplete
ossification of the nasals in S. phaeota. The bony part of each nasal is constricted
in the middle of the long axis of the bone, and the nasals are widest
anteriorly; posteriorly each nasal bears a medial process, which is narrowly
separated from the lateral edge of the ethmoid.
| Table 5.—Variation in the Number of Teeth in the Species of Smilisca. (All Are Males; N = Number of Jaws, or Twice the Number of Individuals; Means Are Given in Parentheses After the Observed Ranges.) | ||||||
| Species | N | Maxillary | Premaxillary | Vomerine | ||
| S. baudini | 20 | 49-65 (56.0) | 9-16 (13.6) | 5-9 (7.2)) | ||
| S. cyanosticta | 8 | 50-64 (57.9) | 10-12 (10.8) | 4-11 (7.1)) | ||
| S. phaeota | 20 | 50-68 (58.1) | 10-15 (12.1) | 5-9 (7.3)) | ||
| S. puma | 6 | 60-67 (63.6) | 11-13 (12.0) | 4-7 (5.3)) | ||
| S. sila | 8 | 48-60 (52.9) | 10-14 (11.3) | 5-7 (5.7)) | ||
| S. sordida | 12 | 39-55 (44.2) | 7-11 (9.3) | 4-6 (5.2) | ||
The teeth of all species of Smilisca are spatulate and bifid. The numbers
of maxillary, premaxillary, and vomerine teeth are summarized in Table 5.
Smaller and presumably younger specimens of all species of Smilisca have
fewer teeth than do larger specimens of the same species. This correlation
[Pg 341]
between size and number of teeth does not exist as an interspecific trend
within the genus; for example, the smallest species in the genus, S. puma,
has the highest number of maxillary teeth. In small specimens of a given
species wide gaps are present between the maxillary teeth posteriorly; in
large specimens the gaps are filled by teeth, beginning anteriorly and progressing
posteriorly, until the maxillary dentition is continuous.
No extensive study of the muscular system was undertaken, but certain
muscles know to be of taxonomic importance were studied.
Jaw Musculature.—Starrett (1960) pointed out the unique jaw musculature
in Smilisca. In this genus M. depressor mandibulae consists of two parts, one
arising from the dorsal fascia and one from the posterior arm of the squamosal.
Two muscles arise from the anterior arm of the squamosal and insert on the
lateral face of the mandible. Of these muscles, M. adductor mandibulae
posterior subexternus lies medial to the mandibular branch of the trigeminal
nerve; the other, M. adductor mandibulae externus superficialis, lies lateral to
the same nerve (Fig. 9). In most other hylids the latter muscle is absent.
No significant variation in the position of the muscles was noted in the various
species of Smilisca, though M. adductor mandibulae originate somewhat more
anteriorly in S. baudini and S. cyanosticta than in the other members of the
genus, all of which have a shorter anterior arm of the squamosal that does not
reach the maxillary. The two separate parts of M. depressor mandibulae are
not so widely separated in members of the sordida group as in the baudini
group.
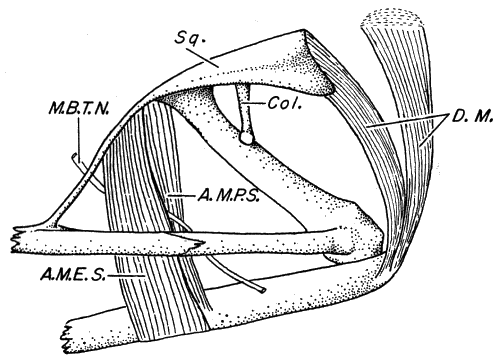
adductor mandibulae externus superficialis; A. M. P. S., adductor mandibulae
posterior subexternus; Col., columella; D. M. depressor mandibulae;
M. B. T. N., mandibular branch trigeminal nerve; Sq., squamosal.
KU 64214, ×5.
[Pg 342]
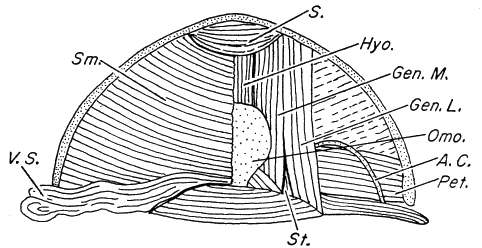
baudini (Superficial musculature on left, deep musculature on
right); A. C. anterior cornua of hyoid; Gen. L., geniohyoideus lateralis;
Gen. M., geniohyoideus medialis; Hyo., hyoglossus; Omo., omosternum;
Pet., petrohyoideus; S., submentalis; Sm., submaxillaris; St.,
sternohyoideus; V. S., vocal sac. KU 64220, × 2.5.
Throat Musculature.—The frogs that comprise the genus Smilisca are
characterized by paired subgular vocal sacs, essentially the same as those in
Triprion (Duellman and Klaas, 1964). The following description is based
on Smilisca baudini (Fig. 10).
M. submentalis lies in the anterior angle of the lower jaw, is thick, and
consists of transverse fibers extending between the dentaries. M. submaxillaris
is thin and arises from the whole of the inner surface of the lower jaw, except
for the anterior angle occupied by M. submentalis. Anteriorly M. submaxillaris
is broadly attached by fascia to M. hyoglossus and M. geniohyoideus,
which lie dorsal to M. submaxillaris. Medially this attachment continues
posteriorly for about one-half the length of the hyoglossus. Posteriorly M.
submaxillaris is folded and attached to M. sternoradialis of the pectoral girdle.
The vocal sacs are formed by a pair of posterolateral evaginations of M.
submaxillaris; a broad connection between the pouches allows free passage
of air between the pouches.
The deeper throat musculature is essentially the same as that described
for Phrynohyas spilomma by Duellman (1956), except for slight differences
in the place of attachment on the hyoid.
Structure
The skin of Smilisca is typical of that of most hylids in organization and
structure. Smilisca sila is distinguished from other members of the genus by
the presence of small wartlike protrusions and peculiar white, pustular spots
on the dorsum. The wartlike structures are composed of three or four
epidermal cells, which protrude from the surface of the epidermis; the structures
are covered by a slightly thickened layer of keratin. The white pustules are
slightly elevated above the surrounding skin. Internally they consist of aggregations
[Pg 343]
of swollen, granular, pigment-cells (perhaps lipophores) lying between
the epidermis and the melanophores.
Biochemical Variations
Dried skins of all species of Smilisca were sent to José M. Cei, Instituto
Nacional de Cuyo, Mendoza, Argentina, for biochemical screening by means
of the chromatographic techniques described by Erspamer and Cei (1963).
The species in the baudini group have detectable amounts of penta-hydroxi-trypatamine,
whereas only a trace is present in the other species. Furthermore,
species in the baudini group differ from S. sila and the sordida group in lacking,
or having only a trace of, tryptophan-containing polypeptides. These
superficial biochemical tests support the arrangement of species as ascertained
by conventional taxonomic characters.
External Morphological Characters
The features of external morphology that were studied in connection with
the taxonomy of the genus Smilisca are discussed below.
Size and Proportions
The frogs of the genus Smilisca are medium to large tree frogs. The three
species comprising the baudini group (S. baudini, cyanosticta, and phaeota)
are notably larger than S. puma, sila, and sordida (Table 6). The largest
specimen that we examined is a female of S. baudini having a snout-vent
length of 90 mm. Smilisca puma is the smallest species; the largest male has
a snout-vent length of 38 mm. and the largest female, 46 mm.
| Table 6.—Comparison of Sizes and Certain Proportions of the Species of Smilisca. (Means in Parentheses Below Observed Ranges; Data for Males Only.) | ||||
| Species | N | Snout-vent length | Tibia length/ snout-vent | Tympanum/ eye |
| S. baudini | 140 | 47.3-75.9 | 42.1-53.6 | 56.1-94.4 |
| (58.7) | (47.8) | (73.5) | ||
| S. cyanosticta | 40 | 44.6-56.8 | 51.9-59.7 | 62.7-88.4 |
| (50.7) | (56.0) | (71.4) | ||
| S. phaeota | 50 | 40.8-65.5 | 50.9-60.2 | 62.7-85.5 |
| (53.9) | (55.5) | (76.6) | ||
| S. puma | 20 | 31.9-38.1 | 48.2-53.1 | 52.1-72.2 |
| (34.7) | (51.3) | (64.9) | ||
| S. sila | 33 | 31.6-44.8 | 49.7-58.1 | 47.6-58.3 |
| (37.7) | (54.8) | (53.2) | ||
| S. sordida | 55 | 31.9-44.6 | 50.5-57.1 | 46.5-57.1 |
| (37.9) | (53.4) | (49.1) | ||
No outstanding differences in proportions exist between species, although
[Pg 344]
certain proportions are sufficiently different in some species to warrant mention.
Smilisca baudini is a more squat and stocky frog than other members of
the genus; this is reflected in the somewhat shorter hind legs (Table 6). The
size of the tympanum relative to that of the eye is highly variable within
samples of a given species. Even so, noticeable differences in the tympanum/eye
ratio are apparent. Members of the baudini group have the largest
tympani, whereas S. sila and sordida have the smallest, and S. puma is intermediate
(Table 6).
Shape of Snout
Although all members of the genus have rather truncate snouts, subtle differences
exist among the species (Pl. 12). Smilisca sila has the shortest snout;
that of S. baudini is only slightly longer. The snouts of S. cyanosticta and
puma are nearly square in lateral profile, whereas those of S. phaeota and
sordida are slightly inclined. The shape of the snout is relatively uniform
within each species and displays no noticeable sexual dimorphism, except in
S. sordida, in which there are sexual differences and geographic variation
(see p. 324).
Hands and Feet
The characters of the hands and feet are among the most taxonomically
important external features in Smilisca. Consistent differences exist in relative
lengths of the digits, size of subarticular tubercles, size and number of supernumerary
tubercles, size and shape of the inner metatarsal tubercle, and
amount of webbing (Pls. 4 and 5). In the baudini group the series of species
(baudini-phaeota-cyanosticta) show a progressive increase in amount of webbing
in the hand and a decrease in number, and corresponding increase in
size, of supernumerary tubercles. The amount of webbing in the feet of
S. baudini and phaeota is about the same, but the webbing is slightly more
extensive in S. cyanosticta. Smilisca puma is unique in the genus in lacking
webbing in the hand; furthermore, this species is distinctive in having many
large subarticular tubercles on the hand and a relatively small inner metatarsal
tubercle. The two stream-inhabitants, S. sila and sordida, have shorter and
stouter fingers than the other species. The webbing is most extensive in both
the hands and feet of these species, which also are distinctive in having many
small supernumerary tubercles on the feet.
Ontogenetic Changes
Minor ontogenetic changes in structure involve the shape of the snout,
relative size of the eye, development of the tympanum, and amount of webbing
in the hand. In recently metamorphosed young the snout is more
rounded than in adults; the canthus and loreal concavity are not evident.
Usually the tympanum is not differentiated in recently metamorphosed young,
and the eye is proportionately large. The webbing in the feet is completely
developed at metamorphosis, but young individuals have noticeably less webbing
in the hand than do adults of the same species.
Coloration
Some of the most distinctive characters of the species of Smilisca are color
and pattern of the living frogs. Although many chromatic features are lost or
subdued in preserved specimens, the patterns usually persist.
[Pg 345]
Metachrosis
Change in color, well known in frogs, is common in hylids, especially in
species having green dorsal surfaces (Phyllomedusa is a notable exception).
The non-green Smilisca (puma, sila, and sordida) changes color, but this
mostly is a change in intensity of color. In these species the markings usually
are most distinct at night; frequently by day the frogs become pallid. The
most striking examples of metachrosis in Smilisca are found in the baudini
group, in which the dorsal ground-color changes from green to tan; correlated
with the change in ground-color may be a corresponding change in the dorsal
markings, but the dorsal markings may change to the opposite color.
Chromosomes
Chromosomes of all six species of Smilisca were studied by means of the
propriono-orcein squash technique described by Duellman and Cole (1965).
Karyotype analysis was attempted for several species by means of intraperitoneal
injections of colchicine, which affected the mitotic cells as desired, but
the testes examined contained too few mitotic cells to allow accurate determination
of karyotypes.
Haploid (n) chromosome numbers were determined from cells in diakinesis,
metaphase I, and metaphase II of meiosis. Diploid (2n) chromosome numbers
were determined from cells in late prophase and metaphase of mitosis.
Chromosome counts from as few as 23 meiotic cells of S. phaeota and as many
as 80 cells of S. sordida reveal a constant haploid (n) number of 12; counts
of chromosomes in one to five mitotic cells in all species, except S. sila, reveal
that the diploid (2n) number is 24.
Breeding
Like most hylid frogs Smilisca is most readily collected and observed when
individuals congregate for breeding.
Time of Breeding
Smilisca breeds primarily in quiet water and reaches its height of breeding
activity at times of plentiful rainfall,—usually from May through October.
Through most of its range Smilisca baudini breeds in those months, but in
some places where abundant rain falls in other seasons, the species breeds at
those times. For example, in southern El Petén and northern Alta Verapaz,
Guatemala, Smilisca baudini has been found breeding in February and March.
The other pond-breeding species (S. cyanosticta, phaeota, and puma) live in
regions lacking a prolonged dry season, and possibly they breed throughout
the year, but breeding activity seems to be greatest in the rainiest months.
The two stream-breeding species (S. sila and sordida) breed in the dry
season when the streams are low and clear, principally in December through
April. At high elevations the species sometimes breed in the rainy season;
also, individuals sometimes breed in the short dry season (summer canicula)
in July and August.
[Pg 346]
At several localities species have been found breeding at different times of
the year: S. baudini in March and July at Chinajá, Guatemala; S. phaeota in
April and August at Palmar Sur, Costa Rica; S. puma in February and July
at Puerto Viejo, Costa Rica; and S. sila in February, April, and August at
El Volcan, Panamá. These observations indicate only that the population
breeds at more than one time in the year, but do not provide any evidence on
the breeding cycles of the individual frogs. This is one important aspect of
the natural history of Smilisca for which we lack data.
Breeding Sites
All members of the genus Smilisca presumably deposit their eggs in water.
Smilisca baudini usually breeds in temporary rain pools; often these are
nothing more than shallow, muddy puddles. In other instances the sites are
extensive ditches or large flooded areas (Pl. 8, Fig. 1). This species is an
opportunistic breeder, and males gather at any of a wide variety of suitable
breeding sites that are formed by torrential rains in the early part of the
rainy season. Smilisca baudini nearly always breeds in open pools having bare
earthen edges. Frequently congregations of S. baudini are found at such
small pools, but are absent from nearby large ponds surrounded by vegetation.
Little is known of the breeding habits of S. cyanosticta, which inhabits
humid forests on foothills and lowlands. Apparently its breeding sites are
not unlike those of S. phaeota, which usually are pools surrounded by vegetation
(Pl. 8, Fig. 2), although sometimes males of S. cyanosticta call from
open muddy puddles. In uplands, where standing water is uncommon, this
species breeds in quiet pools in streams.
Smilisca puma breeds in grass-choked ponds and marshes, where the males
call from bases of dense clumps of grass in the water (Pl. 9, Fig. 1).
Smilisca sila and S. sordida differ noticeably from other species in the genus
by breeding exclusively in streams, where males usually call from rocks or
gravel bars in or at the edges of streams (Pl. 9, Fig. 2); sometimes individuals
perch on bushes overhanging streams. In the streams, or parts of streams,
utilized by these frogs the water is clear, shallow, and has a slow gradient;
occasional males have been found calling along cascading mountain streams.
Breeding choruses composed of ten or more species of frogs are not uncommon
in Middle America, but Smilisca usually breeds alone or with one
or two other species and at the most five others. This tendency towards solitary
breeding possibly is the result of selection of breeding sites that are
unsuitable to many other species of frogs. Nevertheless, many other species
of frogs have been found at the breeding sites with the various species of
Smilisca; these breeding associates (Table 7) are most numerous for S.
baudini, which has a broad geographic range, including a variety of habitats.
Breeding Behavior
Calling sites.—All species of Smilisca usually call from the ground, including
rocks and gravel bars; some individuals sit in shallow water near the edge of
the pool or stream. Sometimes males of S. baudini, sila, and sordida call from
low bushes or trees near the breeding site. One S. baudini was observed calling
while it was floating on the surface of a pond. Smilisca cyanosticta,
phaeota, and puma call from secluded places at the edge of the water or in
the water, whereas S. baudini, sila and sordida call from open situations.
[Pg 347]
| Table 7.—Breeding Associates of the Various Species of Smilisca. | ||||||
| Associate | S. baudini | S. cyanosticta | S. phaeota | S. puma | S. sila | S. sordida |
| Rhinophrynus dorsalis | X | — | — | — | — | — |
| Leptodactylus bolivianus | — | — | X | — | — | — |
| Leptodactylus labialis | X | — | X | — | — | — |
| Leptodactylus melanonotus | X | — | X | X | X | — |
| Leptodactylus occidentalis | X | — | — | — | — | — |
| Leptodactylus quadrivittatus | — | — | X | — | — | — |
| Leptodactylus pentadactylus | — | — | X | X | — | X |
| Engystomops pustulosus | X | — | X | — | — | — |
| Bufo canaliferus | X | — | — | — | — | — |
| Bufo cavifrons | — | X | — | — | — | — |
| Bufo coccifer | X | — | — | — | — | — |
| Bufo coniferus | — | — | X | — | — | — |
| Bufo cristatus | — | X | — | — | — | — |
| Bufo gemmifer | X | — | — | — | — | — |
| Bufo haematiticus | — | — | X | — | X | X |
| Bufo kellogi | X | — | — | — | — | — |
| Bufo luetkeni | X | — | — | — | — | — |
| Bufo marinus | X | — | X | X | X | X |
| Bufo marmoreus | X | — | — | — | — | — |
| Bufo mazatlanensis | X | — | — | — | — | — |
| Bufo melanochloris | — | — | X | — | X | X |
| Bufo perplexus | X | — | — | — | — | — |
| Bufo typhonius | — | — | X | — | X | — |
| Atelopus varius | — | — | — | — | X | X |
| Diaglena reticulata | X | — | — | — | — | — |
| Diaglena spatulata | X | — | — | — | — | — |
[Pg 348]
| Table 7.—Continued | ||||||
| Associate | S. baudini | S. cyanosticta | S. phaeota | S. puma | S. sila | S. sordida |
| Hyla boulengeri | — | — | X | — | — | — |
| Hyla colymba | — | — | — | — | X | — |
| Hyla ebraccata | X | — | X | — | — | — |
| Hyla elaeochroa | — | — | X | X | — | — |
| Hyla eximia | X | — | — | — | — | — |
| Hyla legleri | — | — | — | — | — | X |
| Hyla microcephala | X | — | X | — | — | — |
| Hyla phlebodes | — | — | X | X | — | — |
| Hyla picta | X | — | — | — | — | — |
| Hyla robertmertensi | X | — | — | — | — | — |
| Hyla rosenbergi | — | — | X | — | — | — |
| Hyla rufioculis | — | — | — | — | — | X |
| Hyla smithi | X | — | — | — | — | — |
| Hyla staufferi | X | — | — | — | — | — |
| Hyla walkeri | X | — | — | — | — | — |
| Phrynohyas inflata | X | — | — | — | — | — |
| Phrynohyas spilomma | X | — | — | — | — | — |
| Phrynohyas venulosa | X | — | — | — | — | — |
| Phyllomedusa callidryas | X | — | X | — | — | — |
| Phyllomedusa dacnicolor | X | — | — | — | — | — |
| Phyllomedusa moreleti | X | X | — | — | — | — |
| Pternohyla fodiens | X | — | — | — | — | — |
| Smilisca baudini | X | X | — | — | — | — |
| Smilisca cyanosticta | X | X | — | — | — | — |
| Smilisca phaeota | — | — | X | — | — | — |
| Smilisca puma | — | — | — | X | — | — |
[Pg 349]
| Table 7.—Concluded | ||||||
| Associate | S. baudini | S. cyanosticta | S. phaeota | S. puma | S. sila | S. sordida |
| Smilisca sila | — | — | — | — | X | X |
| Smilisca sordida | — | — | X | — | X | X |
| Triprion petasatus | X | — | — | — | — | — |
| Cochranella fleischmanni | — | — | — | — | X | X |
| Centrolene prosoblepon | — | — | — | — | X | — |
| Gastrophryne elegans | X | — | — | — | — | — |
| Gastrophryne olivacea | X | — | — | — | — | — |
| Gastrophryne usta | X | — | — | — | — | — |
| Hypopachus alboventer | X | — | — | — | — | — |
| Hypopachus caprimimus | X | — | — | — | — | — |
| Hypopachus inguinalis | X | — | — | — | — | — |
| Hypopachus maculatus | X | — | — | — | — | — |
| Hypopachus oxyrrhinus | X | — | — | — | — | — |
| Hypopachus variolosus | X | — | — | — | — | — |
| Rana palmipes | X | — | X | X | — | — |
| Rana pipiens | X | — | — | — | — | — |
| Rana warschewitschi | — | — | X | — | X | X |
Chorus structure.—Limited observations on some of the species of Smilisca
show a definite organization of the calling behavior of individuals. Smilisca
baudini and S. phaeota call in duets. This is especially noticeable in S. baudini,
in which the members of a duet often call from sites separated by only a few
centimeters. The call of S. baudini consists of a series of like notes (see
description of call in following section); the duration of each note is about
equal to the interval between notes. Normally one individual utters one note,
pauses, and utters a single note again, or series of two or three notes. If
there is no response, the first individual often waits several seconds or even
several minutes and then repeats the call. The second individual usually
responds after the first or second note of the sequence. The notes of the second
individual usually are spaced so that they are emitted in the intervals between
the notes of the first individual. This can be shown diagrammatically by having
[Pg 350]
the figure "1" represent notes of the first individual and figure "2," the notes
of the second; an empty interval is represented by "0":
1-0-1-2-1-2-1-2-1-2-1-2
Usually a chorus is initiated by one duet and is quickly picked up by other
individuals also calling in duets. A numerical representation of a chorus of
eight frogs would approximate the following organization:
1-0-1-2-1-2-1-2-1-2-1-2-1-2
3-0-3-4-3-4-3-4-3-4-3-4-3
5-6-5-6-5-6-5-6-5-6-5-6
7-8-7-8-7-8-7-8-7-8-7-8
After the first one or two duets are initiated, the second individuals in
the following duets usually call immediately after their respective partners
have given the first notes. The other noteworthy aspect about the organization
is that the entire chorus usually stops abruptly. Normally the first duet
stops calling shortly before the others, but this is not invariable. Often one
duet or one individual will emit several notes after the rest of the frogs have
become silent. An interval of several minutes sometimes elapses before the
chorus begins again. Successive choruses apparently are initiated by the same
duet. Responses can be initiated artificially by imitating the call, and sometimes
any loud noise will start a chorus.
Similar duets have been observed in S. phaeota. In this species the intervals
are often much longer than the notes, and if two males are calling in
close proximity, their calls can be mistaken for those of one individual.
Smilisca phaeota does not congregate in large numbers; usually only two males
call from one restricted site.
Smilisca sila has a call consisting of a primary note followed by one or more
secondary notes. Males often call in duets, but not necessarily so. In a duet,
the first male usually utters only primary notes until the second individual responds;
then each individual produces a rapid series of secondary notes.
Smilisca puma also produces primary and secondary notes. Although individuals
sometimes call alone, duets, trios, or quartets were more common.
The chorus is initiated by one individual uttering primary notes until joined by
the second, third, and fourth frogs. In one quartet in a marsh 7.5 kilometers
west of Puerto Viejo, Costa Rica, on February 19, 1965, the same individual
initiated four consecutive choruses. Each time the second member of the
chorus was the same; the third and fourth frogs joined the chorus nearly
simultaneously.
Individuals of S. sordida are usually irregularly situated along a stream.
No duets or other combinations of individuals are apparent in the chorus
structure, but once an individual calls, a frog nearby calls almost immediately;
then a frog near the second individual calls, and so on. The resulting series
of calls gives the impression that the sound is moving along the stream as successive
individuals join the chorus and the first callers become quiet. It is
not known if the same individual initiates successive choruses or if the order
of calling is the same in subsequent choruses.
These limited observations on chorus structure in Smilisca show the presence
of behavioral organization. The methods of establishing the organization and
the significance of the call-order in breeding have yet to be discovered.
Calling males of S. baudini are often close together; some individuals have
been observed almost touching one another, but no indication of territoriality
[Pg 351]
or aggressive behavior has been witnessed. The more distant spacing of the
stream-breeding species S. sila and S. sordida may be a function of calling-territories,
but no direct evidence is available to substantiate this supposition.
Sex recognition and amplexus.—Observations on Smilisca baudini indicate
that the calls of males attract females. At Tehuantepec, Oaxaca, México, a
female was first observed about two meters away from a male calling at the
edge of a rain pool; in a series of short hops she progressed directly towards
the male, although vegetation obscured him until she was less than a meter
away. When she approached to within about 20 centimeters of the male,
he took notice of her, moved to her, and clasped her. At Chinajá, Alta
Verapaz, Guatemala, a female swam directly across a pool about three meters
wide to a calling male. Her line of movement took her within a few centimeters
of a silent male, to whom she paid no attention. She stopped just in
front of the calling male, which immediately clasped her. At a large muddy
pond 4 kilometers west-northwest of Esparta, Puntarenas, Costa Rica, a female
was observed swimming toward a small submerged tree; a male was
calling from a branch about one meter above the water. The female climbed
to a branch about 20 centimeters below the male, which upon seeing her
there immediately jumped down and clasped her. These few observations of
S. baudini show that in this species females are capable of locating calling
males by means of phono-orientation; visual reception on the part of females
seems to be secondary. Contrariwise, males apparently become aware of the
proximity of females by seeing them; once a male sees a female he usually
tries to clasp her. Possibly the males receive stimuli by means of chemo-reception,
but in each observed instance the male obviously looked at the
female.
Amplexus is axillary in all members of the genus. Normally amplexing
males hunch their backs and press their chins to the females' backs. Clasping
pairs are usually found at the edge of the water, but sometimes amplexus
takes place in trees or bushes.
Egg deposition.—Oviposition has been observed only in Smilisca baudini.
On the night of June 28, 1961, at Chinajá, Alta Verapaz, Guatemala, a clasping
pair was observed at the edge of a shallow rain pool. After sitting for
several minutes in shallow water, the female (with male on her back) swam
part way across the pool and grasped an emergent stick with one hand. The
female's body was nearly level with the surface of the water, and her hind
legs were outstretched as deposition commenced; eggs were extruded rapidly.
After a few seconds the female moved slowly to another twig a few centimeters
away and deposited more eggs. This process was repeated until the
female was spent. The spawn resulted in a surface film covering roughly one
square meter. It is doubtful if this type of egg deposition occurs in any other
species in the genus, especially those that lay their eggs in streams.
Breeding Call
The breeding calls of the six species of Smilisca are alike in their explosive
nature. Calls are emitted quickly with a short burst of air filling the vocal
sac, which immediately deflates. Phonetically the calls can be described as a
single "wonk" or series of such notes in S. baudini and S. cyanosticta, a low
growl in S. phaeota, a relatively high pitched rattle in S. sordida, and a low
[Pg 352]
squawk usually followed by one or more rattling secondary notes in S. puma
and S. sila. Quantitatively, the calls of the six species differ in number of
notes, duration of notes, and in pitch (Table 8, Pls. 10 and 11). Although
no measurements were taken on the intensity of the calls, we observed in the
field that each of the species has a loud voice. The call of S. baudini seems
to carry farther than any of the others.
| Table 8.—Comparison of Breeding Calls in Smilisca. (Observed Range Given in Parentheses Below Mean. In Species Having Primary and Secondary Notes, Only the Primary Notes Are Analyzed Here.) | |||||||
| Species | N | Notes per call group | Duration of note (seconds) | Pulses per second | Fundamental frequency (cps) | Major frequencies (cps) | |
| Lower | Upper | ||||||
| S. baudini | 20 | 8.0 | 0.11 | 174.7 | 166.2 | 351 | 2507 |
| (2-15) | (0.09-0.13) | (140-195) | (135-190) | (175-495) | (2400-2725) | ||
| S. cyanosticta | 10 | 1.2 | 0.38 | 147.0 | 145.1 | 841 | 1894 |
| (1-2) | (0.25-0.45) | (110-180) | (135-160) | (480-975) | (1600-2100) | ||
| S. phaeota | 10 | 1.6 | 0.31 | 116.0 | 143.0 | 372 | — |
| (1-2) | (0.10-0.45) | (100-130) | (110-165) | (330-495) | |||
| S. puma | 28 | 3.7 | 0.13 | 208.2 | 145.6 | 743 | 1868 |
| (2-10) | (0.06-0.35) | (187-240) | (125-200) | (495-980) | (1456-2240) | ||
| S. sila | 15 | 2.4 | 0.16 | 108.5 | 103.0 | 899 | 2218 |
| (1-6) | (0.06-0.28) | (97-120) | (90-115) | (665-1180) | (1980-2700) | ||
| S. sordida | 19 | 1.7 | 0.29 | 104.7 | 123.1 | 1216 | 2694 |
| (1-6) | (0.18-0.45) | (78-135) | (90-140) | (1150-1540) | (2340-2990) | ||
[Pg 353]
Call rate.—The rate at which call-groups are produced varies from one
every few seconds to one in several minutes. In S. baudini, cyanosticta, phaeota,
and sordida, call-groups are produced as frequently as every 12 seconds,
but usually more time elapses between call groups. In S. sordida, five or more
minutes sometimes elapse between call-groups. The interval is somewhat less
in S. phaeota. Calls are repeated at much shorter intervals in S. puma (5-55
seconds) and S. sila (4-20 seconds).
Notes per call-group.—Except for S. puma and S. sila, the series of notes
produced in any given call of a species of Smilisca is essentially the same;
there is no differentiation into primary and secondary notes. Smilisca cyanosticta
and S. phaeota emit only one or two relatively long notes per call-group,
whereas S. baudini and S. sordida produce as many as 15 and 6 notes,
respectively. Males of S. puma and S. sila often produce only the primary
note; sometimes this is done several times before the secondary notes are produced.
For example, one S. puma (KU 91711; tape No. 379) produced the
following number of notes in consecutive call-groups: 1, 1, 1, 1, 2, 2, 3, 1, 4;
secondary notes are present in only four of the nine call-groups. A typical
series of consecutive call-groups in S. sila (KU 91852; Tape No. 385) has
1, 1, 1, 2, 4, 2 notes per call-group; secondary notes are present in only half
of the call-groups. Smilisca puma apparently always produces at least two
primary notes before emitting secondary notes; sometimes only primary notes
are produced in one series of calls. The number of secondary notes following
a given primary varies from one to nine; the modal number is one, and the
mean is three in 27 call-groups. Smilisca sila frequently begins a series of calls
with two or more primary notes, but sometimes the first primary note is followed
immediately by two or more secondary notes. The number of secondary
notes following a given primary varies from one to five; the modal number is
one, and the average is two in 13 call-groups.
Duration.—The average duration of call-groups consisting of two or more
notes is 1.18 seconds in S. baudini; 1.02 in cyanosticta, 0.91 in phaeota, 1.32
in puma, 1.48 in sila, and 1.29 in sordida. Although there is considerable
variation in the lengths of the notes (only primary notes in S. puma and sila
are considered here), S. cyanosticta, phaeota, and sordida have noticeably
longer notes than do the other species (Table 8). The secondary notes are
longer than the primary notes in S. puma (average 0.27 secs. as compared with
0.13 secs.) and in S. sila (average 0.25 secs., as compared with 0.16 secs.).
Note repetition rate.—The rate at which notes in call-groups containing two
or more notes are produced varies in S. baudini from 2.5 to 7.1 (average, 3.7)
calls per second; cyanosticta, 1.8-2.1 (1.9); phaeota, 2.0-2.4 (2.2); puma,
1.9-2.9 (2.2); sila, 1.3-2.4 (1.8); and sordida, 1.5-2.6 (2.1). Smilisca baudini,
which has notes of short duration (0.09 to 0.13 seconds), has the fastest note-repetition
rate. Although the individual notes of S. cyanosticta and S. phaeota
are relatively long (average, 0.38 and 0.31 seconds, respectively), the intervals
[Pg 354]
between the notes is short; consequently, their note-repetition rates do not
differ greatly from those of S. puma and S. sila, which have shorter notes (average,
0.13 and 0.16 seconds, respectively) but longer intervals between notes.
Pulse rate.—Pulses vary in frequency from 78 to 240 per second in the calls
analyzed (only primary notes in S. puma and S. sila), but the variation in any
given species is much less than that in the entire genus (Table 8). Smilisca
puma is outstanding in having a high pulse rate, which is approached only by
that of S. baudini. Even in the species having the lowest pulse rates, the
pulsations are not audible. The secondary notes produced by S. puma and
S. sila have a slower pulse rate than the primary notes; often the pulses are
audible. In S. puma the pulse rate of secondary notes is sometimes as low as
48 pulses per second, and in S. sila still lower (as low as 40 pulses per second).
The upper limits of pulse rate in the secondary notes in these species merge
imperceptibly with the rates of the primary note; consequently, on the basis
of pulse rate alone it is not always possible to distinguish primary from secondary
notes.
Frequency.—Smilisca produces noisy (as opposed to more musical) calls,
and the energy is distributed throughout the frequency spectrum; the calls are
poorly modulated, except in S. sordida, in which two usually discrete bands of
frequency are present (Pl. 11C). For the most part the calls of Smilisca consist
of little modified energy of the fundamental frequency and of its harmonics,
some of which are emphasized.
The upper frequency range varies within each species and even within the
calls of one individual. Smilisca phaeota has the lowest upper frequencies;
no calls ranged above 4400 cycles per second (cps.), and half of the calls
never exceeded 3000 cps. Smilisca cyanosticta produces calls in which the
upper frequency is below 7000 cps. and usually below 6000 cps. Likewise,
S. puma produces calls that are below 7000 cps., whereas S. sila has frequencies
of up to 8400 cps. In both S. baudini and S. sordida, the highest frequencies
attained are about 9100 cps. Variation in the highest frequencies in
a series of consecutive calls by one individual frog was noted in all species.
Such variation is especially prevalent in S. puma; for example one individual
(KU 87771; Tape No. 376) recorded at a temperature of 24° C. at 7.5 kilometers
west of Puerto Viejo, Heredia Province, Costa Rica, on July 31, 1964,
produced three consecutive primary notes having upper frequencies of about
6000, 4000, and 4000 cps., respectively. Apparently in a given species the
production of the higher frequencies in some notes and not in others is correlated
with the amount of distention of the vocal sac and is not dependent
upon the structure or tension of the vocal cords.
Although the dominant frequency in S. sordida is lower than that in S.
baudini and S. cyanosticta, the call of the former is audibly higher-pitched.
This is due primarily to the emphasis on certain harmonics at a high frequency
(sometimes as high as 9000 cps.) in S. sordida, whereas in S. baudini and
other species, if harmonics are present at those frequencies, they are not emphasized.
The fundamental frequencies are as low as 90 cps. in S. sila and S. sordida
and as high as 200 cps. in S. puma (Table 8). The fundamental frequency
seemingly is relatively unimportant in determining the general pitch of the call,
a characteristic most dependent on the dominant frequency and emphasized
harmonics in the higher-frequency spectrum. In none of the species is the
[Pg 355]
fundamental the dominant frequency. In the low-pitched call of S. phaeota
the dominant frequency is the third harmonic (the second harmonic above the
fundamental frequency, which is the first harmonic). In all other species a
much higher harmonic is dominant; for examples, in S. cyanosticta harmonics
from 10 to 15 are dominant; in S. baudini, 15-19; and S. sila, 20-30.
A glance at the audiospectrographs and their accompanying sections (Pls.
10 and 11) reveals the presence of two emphasized bands of frequency in all
species except S. phaeota, in which only the lower band is present. These two
bands of emphasized harmonics are part of a continuous, or nearly continuous,
spread of energy throughout the frequency spectrum, except in S. sordida in
which the bands are usually distinct. As shown in the sections, certain harmonics
in each of the bands are emphasized with nearly equal intensity.
Therefore, with the exception of S. phaeota, the calls of Smilisca are characterized
by two major frequencies, one of which is the dominant frequency and
the other is a subdominant frequency (Table 8). The upper major frequency
is dominant in all calls in S. baudini and S. cyanosticta, but either major frequency
may be dominant in other species. The upper major frequency is
dominant in 65 per cent of calls by S. puma, 87 per cent in S. sila, and 68 per
cent in S. sordida. Individuals of these three species sometimes produce a
series of calls in which the dominant frequency changes from one of the major
frequencies to the other. Four consecutive notes emitted by an individual of
S. sordida recorded 13 kilometers east-northeast of Golfito, Puntarenas Province,
Costa Rica, had dominant frequencies of 910, 1950, and 750 cps., respectively.
In each case, an alternation of major frequencies took place in
respect to dominance. An individual of S. puma from 7.5 kilometers west of
Puerto Viejo, Costa Rica, produced a primary note followed by one secondary
note; each note had major frequencies at 600 and 1800 cps.; the dominant
frequency of the primary note was at 1800 cps., whereas in the secondary note
the dominant frequency was at 600 cps. The difference in emphasis on the
major frequencies is so slight that shift in dominance is not audible.
Effect of temperature on calls.—The present data are insufficient to test
statistically the correlation between temperature and variation within certain
components of the calls in Smilisca, but even a crude graph shows some general
correlations. The widest range of temperatures is associated with the
recordings of S. baudini. Three individuals recorded at a temperature of 30°
C. at Tehuantepec, Oaxaca, had pulse rates of 180 pulses per second and fundamental
frequencies of 160-180 cps., as compared with an individual recorded
at a temperature of 17° C., which had a pulse rate of 140 and a fundamental
frequency of 135 cps. All individuals of S. baudini recorded at higher temperatures
had faster pulse rates and higher fundamental frequencies. Pulse
rates differ in the other species in the genus but less strikingly (probably owing
to narrower ranges of temperatures at which recordings were made). In five
recordings of S. sordida made at 20° C. the pulse rate is 80-90, as compared
with four recordings made at 25° C. having pulse rates of 120-135. Thirteen
recordings of S. sila made at 17° C. have pulse rates of 97-112 (average 105);
one individual recorded at 26° C. has 120 pulses per second. Seemingly no
correlation exists between temperature and other characteristics of the calls,
such as duration and rate of note-repetition.
The breeding call as an isolating mechanism.—Blair (1958), Bogert (1960),
Duellman (1963a), Fouquette
(1960), Johnson (1959), and others have provided
[Pg 356]
evidence that the breeding calls of male hylids (and other anurans)
serve as isolating mechanisms in sympatric species. In summarizing this discussion
of the breeding calls of Smilisca we want to point out what seem to
be important differences in the calls that may prevent interspecific hybridization
in sympatric species of Smilisca.
The genus is readily divided into two species-groups on morphological
characters; this division is supported by the breeding calls. In the species of
the baudini group the calls are unmodulated and lack secondary notes. In the
sordida group the calls either have secondary notes or are modulated.
Smilisca baudini occurs sympatrically with S. cyanosticta and S. phaeota;
where they occur together, both species sometimes breed in like places at the
same time. We are not aware of these species breeding synchronously at exactly
the same site, although S. baudini and S. cyanosticta were calling on the
same nights and less than 100 meters apart in Oaxaca in June, 1964. Regardless
of their respective breeding habits, sympatric species have calls that differ
notably. Except for the higher fundamental and dominant frequencies, the
calls of S. cyanosticta and S. phaeota closely resemble one another, but the
calls of both species differ markedly from that of S. baudini. The geographic
ranges of S. cyanosticta and S. phaeota are widely separated.
The calls of the allopatric species S. puma and S. sila are not greatly different.
Smilisca sordida has a distinctive call and occurs sympatrically with S.
puma and S. sila. In the streams in southern Costa Rica S. sordida and S. sila
breed synchronously, but the high-pitched modulated call of the former is
notably different from the lower, unmodulated call of S. sila.
The data indicate that the calls of related sympatric species differ more
than the calls of related allopatric species. We postulate that these differences
evolved to support the reproductive isolation of the sympatric species. The
data are insufficient to determine geographic variation in the calls and to determine
if differences in the calls are enhanced in areas of sympatry as compared
with the allopatric parts of the ranges.
Other calls.—As stated previously, there is no direct evidence of territoriality
in Smilisca; we have heard no calls that can be definitely identified as territorial.
Single notes of S. baudini, phaeota, and sila have been heard by day,
just prior to rains, or during, or immediately after rains. Such calls can be
interpreted as "rain calls," which are well known in Hyla eximia and Hyla
squirella. Distress calls are known in several species of Rana and in Leptodactylus
pentadactylus; such calls result from the rapid expulsion of air over
the vocal cords and with the mouth open. Distress calls have been heard
from S. baudini. At Charapendo, Michoacán, México, a male that had one hind
limb engulfed by a Leptodeira maculata emitted several long, high-pitched
cries. A clasping pair of S. baudini was found in a bush at the edge of a
marshy stream 2 kilometers northeast of Las Cañas, Guanacaste Province,
Costa Rica. When the pair was grasped, the female emitted a distress call.
Eggs
Eggs of S. baudini, cyanosticta, and phaeota have been found in the field,
and eggs of S. sila have been observed in the laboratory. The eggs of S. puma
and sordida are unknown. Insofar as known, Smilisca baudini is unique in
the genus in depositing the eggs in a surface film. Each egg is encased in a
[Pg 357]
vitelline membrane, but individual outer envelopes are lacking. The eggs are
small; the diameter of recently-deposited eggs is about 1.3 mm. and that of
the vitelline membrane is about 1.5 mm. The eggs of S. cyanosticta and phaeota
are deposited in clumps, and the eggs are larger than those of S. baudini.
Diameters of eggs of S. cyanosticta are about 2.3 mm., and those of the outer
envelopes are about 4.0 mm. Artificially fertilized eggs of S. sila raised in
the laboratory have diameters of about 2.4 mm.; the diameter of the outer
envelopes is about 4.9 mm.
In order to determine the reproductive potential of the six species, ovulated
eggs were removed from females and counted. The numbers of eggs recorded
are: 3 S. baudini—2620, 2940, 3320; 1 S. cyanosticta—910; 3 S. phaeota—1665,
1870, 2010; 1 S. puma—518; 3 S. sila—369, 390, 473; 3 S. sordida—524,
702, 856. These limited data indicate that the large species (S. baudini,
cyanosticta, and phaeota) have more eggs than do the smaller species. The
stream-breeding species (S. sila and sordida) have relatively few eggs by comparison
with the pond-breeders. Possibly this is a function of size of eggs
rather than a correlation with the site of egg-deposition.
Tadpoles
The acquisition of tadpoles of all of the species of Smilisca has made possible
the use of larval characters in erecting a classification and in estimating
the phylogenetic relations of the several species. Furthermore, developmental
series of tadpoles of four species allow a comparison of the growth and development
in these species. Throughout the discussion of tadpoles we have
referred to the various developmental stages by the Stage Numbers proposed
by Gosner (1960).
General Structure
Tadpoles of the genus Smilisca are of a generalized hylid type, having 2/3
tooth-rows, unspecialized beaks, mouth partly or completely bordered by papillae,
lateral fold present in the lips, spiracle sinistral, anal tube dextral, and
caudal musculature extending nearly to tip of caudal fin. Although minor
differences exist in coloration, proportions, and mouthparts, no great modifications
of the basic structure are present.
Comparison of Species
The larval characters of the species of Smilisca are compared below and
illustrated in Figures 11-15.
Shape and Proportions.—The bodies of S. baudini, cyanosticta, phaeota, and
puma are rounded and about as wide as deep; the eyes are moderately large
and directed dorsolaterally, and the nostrils are about midway between the
bluntly rounded snout and the eyes. The mouths are medium-sized and directed
anteroventrally. The bodies of tadpoles of S. sila and sordida are
slightly compressed dorso-ventrally. The snout is moderately long and sloping;
the eyes are larger and directed more dorsally than in the other species, and
the nostrils are closer to the eyes than the snout. The mouths are moderately
large and directed ventrally.
The tail is about half again as long as the body in S. baudini, cyanosticta,
[Pg 358]
phaeota, and puma; in these species the caudal musculature is moderately
heavy, and the caudal fins are deep. The caudal musculature is upturned
distally in S. baudini and phaeota, and the dorsal fin extends anteriorly onto
the body in these two species and in S. puma. The tail is about twice as long
as the body in S. sila and sordida. In both species the caudal fins are shallow
in comparison with the depth of the caudal musculature, especially in S. sordida
(Fig. 14); in neither species does the dorsal fin extend anteriorly onto the
body.
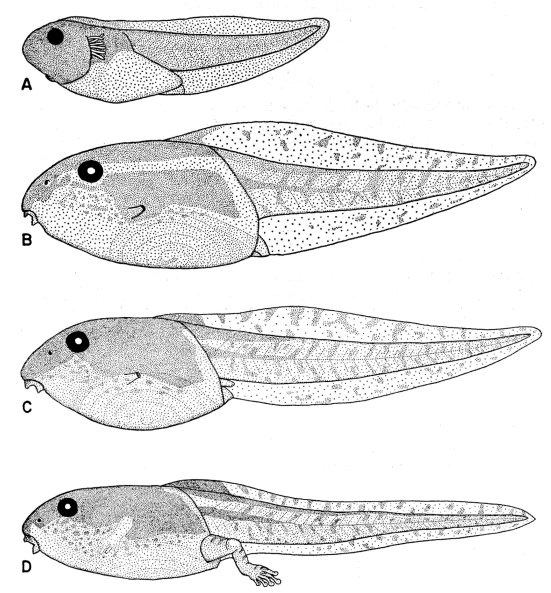
Stage 25 (KU 68467) × 5; (C) Stage 30 (KU 60018) × 4; (D) Stage 41
(KU 60018) × 3.
Mouthparts.—The mouth of S. sordida is completely bordered by two rows
of papillae, whereas in the other species the median part of the upper lip is
devoid of papillae. Smilisca baudini and puma have two rows of papillae; S.
sila has one complete row (except medially on the upper lip) and one incomplete
row, and S. cyanosticta and phaeota have only one row (Fig. 15). All
species have numerous papillae in the lateral fold; the fewest lateral papillae
[Pg 359]
are found in S. cyanosticta and phaeota. Although all species have two rows
of teeth in the upper jaw and three rows in the lower jaw, specific differences
in the nature of the rows exist between certain species. The second upper
tooth-row is narrowly interrupted medially in S. sila and sordida and broadly
interrupted in the other species. The first upper row is strongly arched in S.
puma, moderately arched in S. baudini and sila, and weakly arched in the
other species. In all species the third lower tooth-row is the shortest, only
slightly so in S. sila and sordida, but only about half the length of the second
lower row in S. puma.
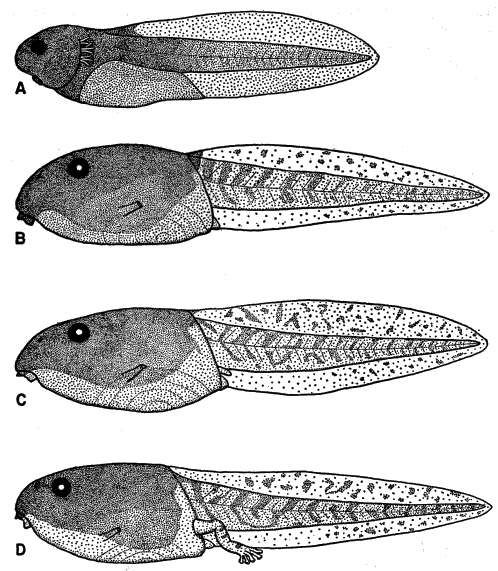
Stage 25 (KU 87651) × 5; (C) Stage 30 (KU 87652) × 4; (D) Stage 40
(KU 87650) × 3.
The beaks are well developed and finely serrate in all species. The lower,
broadly V-shaped, beak is slender in S. puma, rather robust in S. baudini and
[Pg 360]
sila, and moderately heavy in the other species. The lateral processes of the
upper beak are shortest in S. puma and longest in S. baudini and sordida. In
the latter the inner margin of the upper beak and lateral process have the form
of a shallow S, whereas in the other species the inner margin of the upper
beak forms a continuous arch with the lateral processes (Fig. 15).
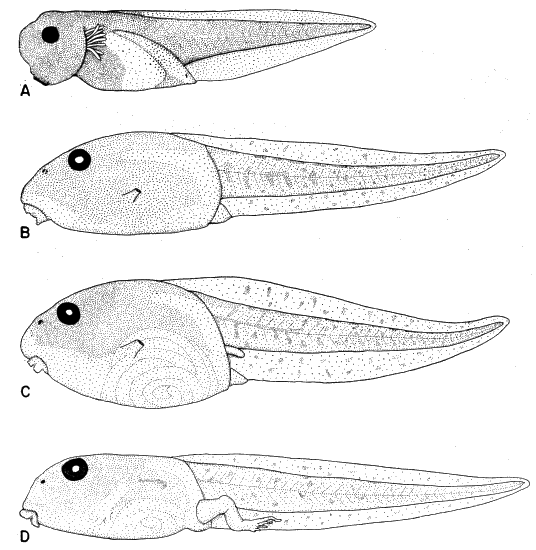
(B) Stage 25 (KU 68480) × 5; (C) Stage 30 (KU 68482) × 4; (D) Stage
40 (KU 68483) × 3.
Coloration.—The tadpoles of Smilisca lack the bright colors or bold markings
characteristic of some hylid tadpoles; even so, the subdued colors and
arrangement of pigments provide some distinctive markings by which the
species can be distinguished from one another. The species comprising the
baudini group (S. baudini, cyanosticta, and phaeota) are alike in having the
body brown or grayish brown dorsally and transparent with scattered brown
pigment ventrally. A cream-colored, crescent-shaped mark is present on the
posterior edge of the body; this mark is usually most noticeable in S. baudini
and least so in S. cyanosticta. Other differences in coloration in members of
the baudini group are relative and subtle. Smilisca phaeota usually is more
[Pg 361]
pallid than baudini, and cyanosticta usually is darker than baudini; both species
have larger dark markings on the tail than does S. phaeota. Smilisca
baudini has a dark streak on the middle of the anterior one-fourth of the tail
(Figs. 11-13).
Smilisca puma is distinctive in having a grayish brown body and dark gray
reticulations on the tail. Smilisca sila and sordida are distinctive in having
pairs (sometimes interconnected) of dark marks on the dorsal surfaces of the
caudal musculature, and in dorsal view the tail appears to be marked with
dark and pale creamy tan transverse bars. These dark marks, as well as the
small flecks on the tail, are brown in S. sila and red in sordida. Smilisca sila
has dark brown flecks on the dorsal surface of the body and small white flecks
laterally; these markings are absent in S. sordida (Fig. 14).
Descriptions of the coloration of living tadpoles are given in the accounts
of the species.
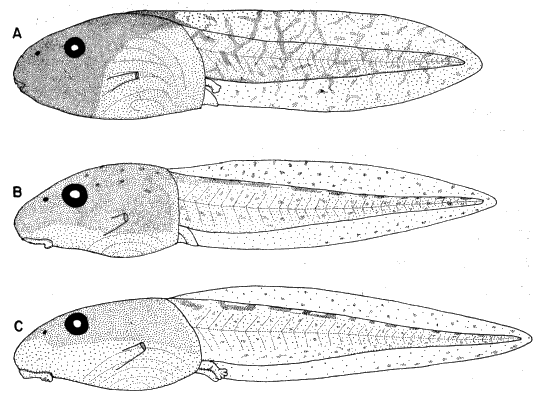
S. sila, Stage 25 (KU 80260); S. sordida, Stage 30 (KU 68475). All × 3.5.
Growth and Development
Information on the growth and development of Middle American hylids is
scanty. Adequate descriptions have been published for Phyllomedusa annae
(Duellman, 1963b), Phrynohyas venulosa (Zweifel, 1964), and Triprion petasatus
(Duellman and Klaas, 1964). Material is available for adequate descriptions
of the developmental stages of four species of Smilisca (Tables 9-12,
Figs. 11-13). Because none of the tadpoles was raised from hatching to metamorphosis,
the rate of growth and duration of the larval stages are unknown.
[Pg 362]
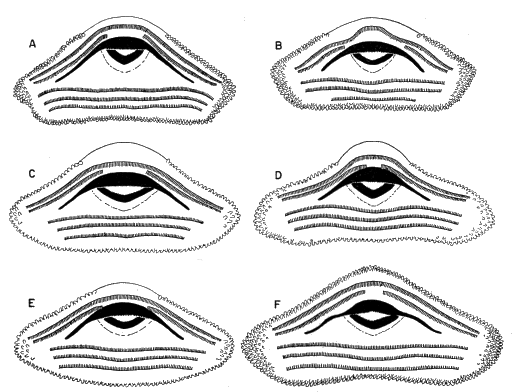
(B) S. puma (KU 91807); (C) S. cyanosticta (KU 87625); (D) S. sila
(KU 80620); (E) S. phaeota (KU 68482); (F) S. sordida (KU 68475).
All ×17.
| Table 9.—Growth and Development of Tadpoles of Smilisca baudini. (Means Are Given in Parentheses After the Observed Ranges.) | ||||
| Stage | N | Total length | Body length | Tail length |
| 21 | 10 | 5.1-5.4 (5.22) | 2.6-2.7 (2.54) | 2.5-2.7 (2.58) |
| 24 | 10 | 6.0-6.5 (6.20) | 2.3-2.6 (2.45) | 3.5-3.9 (3.69) |
| 25 | 10 | 7.2-8.3 (7.78) | 3.0-3.3 (3.14) | 4.2-5.0 (4.64) |
| 27 | 10 | 18.5-21.5 (20.22) | 8.0-9.0 (8.38) | 10.4-13.0 (11.84) |
| 29 | 10 | 21.5-24.5 (22.60) | 8.5-10.0 (9.25) | 12.5-14.5 (13.35) |
| 37 | 3 | 28.5-31.0 (30.00) | 11.0-12.5 (11.67) | 17.5-19.0 (18.00) |
| 38 | 10 | 35.0-37.5 (35.50) | 12.0-13.5 (12.80) | 21.5-24.0 (22.70) |
| 40 | 2 | 34.0-37.0 (35.50) | 12.5-13.5 (13.00) | 21.5-23.5 (22.50) |
| 41 | 10 | 34.0-37.0 (35.50) | 12.5-13.5 (13.00) | 21.5-23.5 (22.50) |
| 42 | 3 | 24.0-30.0 (27.00) | 12.5-13.0 (12.67) | 11.5-17.0 (14.33) |
| 45 | 6 | 14.0-24.0 (17.58) | 12.5-14.0 (13.37) | 1.5-10.0 (4.17) |
| 46 | 23 | —— | 12.0-15.5 (13.34) | —— |
[Pg 363]
| Table 10.—Growth and Development of Tadpoles of Smilisca cyanosticta. (Means Are Given in Parentheses After the Observed Ranges.) | ||||
| Stage | N | Total length | Body length | Tail length |
| 21 | 10 | 5.8-6.5 (6.28) | 2.8-3.1 (3.00) | 3.0-3.5 (3.28) |
| 25 | 10 | 7.9-9.2 (8.44) | 2.7-3.2 (2.96) | 4.8-6.0 (5.48) |
| 30 | 7 | 22.5-25.0 (23.50) | 8.5-9.5 (9.00) | 14.0-15.5 (14.57) |
| 36 | 10 | 27.0-30.0 (28.75) | 9.5-11.5 (10.80) | 17.0-18.5 (17.95) |
| 42 | 2 | 26.0-27.0 (26.50) | 10.00 | 16.0-17.0 (16.50) |
| 46 | 2 | — | 14.00 | — |
Hatchlings of three species (S. baudini, cyanosticta, and phaeota) are available.
These larvae have non-functional eyes and large oral suckers. By the
time the larvae have developed to stage 21, external gills are present, the
caudal musculature and caudal fin have been differentiated, and the head is
distinguishable from the body. In stage 21 oral suckers and a large amount of
yolk are still present.
The developmental data on the four species show no significant variations;
consequently, we will describe the development of only one species, Smilisca
phaeota (Table 11, Figs. 13 and 16).
Stage 21.—Bulging cream-colored yolk mass, transparent cornea, and moderately
long, unbranched filamentous gills, and oral suckers present; mouth
having irregular papillae on lower lip; teeth and beaks absent; caudal myomeres
distinct; pigmentation uniform over body and caudal musculature; caudal
fin transparent with scattered small flecks.
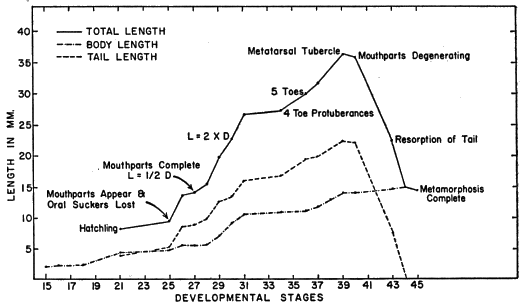
with developmental stages. Formulas for the limb bud refer to its length (L)
in relation to basal diameter (D).
Stage 25.—Operculum complete; gills absent; sinistral spiracle apparently
functional; cloacal tail-piece, nasal capsules, and external nares present; gut
[Pg 364]
partly formed; mouth bordered by single row of papillae, except medially;
small papillae present in lateral fold of lips; two upper and three lower tooth-rows
present, but not fully developed; beaks apparently fully developed; depth
of dorsal and ventral fins less than depth of caudal musculature: tip of tail
upturned; pigment on body most dense on dorsum and sides; faint, nearly
pigmentless crescent-shaped mark on posterior edge of body; concentrations of
pigment forming small spots on tail.Stage 28.—Mouthparts complete; limb bud about half as long as thick;
other structural features and coloration closely resemble those in stage 25.Stage 30.—Limb bud approximately twice as long as thick; body as deep
as wide; dorsal fin deepest just posterior to body; ventral fin deeper than caudal
musculature; tail sharply upturned distally; anal tube dextral; brown pigment
sparse on flanks.
| Table 11.—Growth and Development of Tadpoles of Smilisca phaeota. (Means Are Given in Parentheses After the Observed Ranges.) | ||||
| Stage | N | Total length | Body length | Tail length |
| 15 | 10 | — | 1.9-2.1 (1.97) | — |
| 16 | 8 | — | 2.0-2.2 (2.07) | — |
| 18 | 4 | — | 2.2-2.6 (2.31) | — |
| 21 | 3 | 7.9-8.6 (8.21) | 4.1-4.5 (4.31) | 3.8-4.1 (3.92) |
| 25 | 10 | 8.7-10.6 (9.69) | 4.5-4.8 (4.64) | 4.3-6.0 (5.05) |
| 26 | 11 | 12.3-16.1 (14.01) | 4.2-6.3 (5.60) | 6.7-9.8 (8.41) |
| 27 | 10 | 13.0-15.7 (14.28) | 4.9-6.2 (5.40) | 7.7-10.5 (8.88) |
| 28 | 13 | 13.9-20.9 (15.62) | 5.2-8.3 (5.75) | 8.5-12.6 (9.85) |
| 29 | 8 | 17.8-22.3 (19.79) | 6.3-8.4 (7.19) | 11.5-14.0 (12.60) |
| 30 | 9 | 20.3-24.8 (22.85) | 8.1-10.5 (9.32) | 10.5-15.5 (13.53) |
| 31 | 5 | 24.1-28.5 (26.61) | 9.4-11.2 (10.59) | 14.7-17.3 (16.02) |
| 34 | 5 | 24.8-29.4 (27.31) | 9.2-11.6 (10.73) | 15.6-18.5 (16.80) |
| 36 | 3 | 30.0-30.1 (30.07) | 10.1-12.2 (11.15) | 18.9-20.0 (19.44) |
| 37 | 4 | 28.9-34.1 (31.75) | 11.5-12.4 (11.88) | 17.4-22.5 (19.88) |
| 38 | 1 | 28.98 | 12.88 | 16.10 |
| 39 | 2 | 35.6-36.9 (36.25) | 14.00 | 21.6-22.9 (22.25) |
| 40 | 2 | 32.3-39.8 (36.05) | 14.00 | 18.3-21.8 (20.05) |
| 43 | 2 | 21.5-23.0 (22.25) | 14.2-14.8 (14.45) | 6.8-8.8 (7.80) |
| 44 | 4 | — | 14.5-15.6 (15.08) | — |
| 46 | 11 | — | 12.7-16.7 (14.26) | — |
[Pg 365]
| Table 12.—Growth and Development of Tadpoles of Smilisca sordida. (Means Are Given in Parentheses After the Observed Ranges.) | ||||
| Stage | N | Total length | Body length | Tail length |
| 25 | 6 | 25.5-28.0 (26.1) | 9.0-9.5 (9.3) | 16.2-18.5 (16.7) |
| 33 | 2 | 28.5-30.0 (29.3) | 10.2-10.5 (10.4) | 18.0-19.8 (18.9) |
| 36 | 8 | 29.5-34.5 (32.3) | 10.2-11.7 (10.8) | 19.3-23.0 (21.5) |
| 37 | 7 | 31.6-37.5 (34.6) | 11.0-12.5 (11.5) | 21.6-25.0 (23.2) |
| 41 | 3 | 33.0-37.2 (35.2) | 11.6-12.2 (11.9) | 21.4-25.2 (23.2) |
| 43 | 1 | —— | 12.4 | —— |
| 46 | 9 | —— | 13.1-15.7 (14.9) | —— |
Stages 34, 36, 37, and 38.—Stage 34, foot paddle-shaped with four toe
buds; stage 36, five toe buds; stages 37 and 38, lengthening of toes. In all
four stages, spiracle persistent, and pigmentation resembling that of early
stages.Stage 39.—Metatarsal tubercle present; greatest total length (36.9 mm.)
attained.Stage 40.—Subarticular tubercles prominent; skin over forelimbs transparent;
cloacal tail-piece and spiracle absent; outer tooth-rows degenerating; caudal
fins shallower than in preceding stages; distal part of tail nearly straight;
size of dark markings on tail decreased; pigment present on hind limb.Stage 43.—Forelimbs erupted; larval mouthparts absent; corner of mouth
between nostril and eye; transverse bands present on hind limbs; tail greatly
reduced (about 8 mm. in length).Stage 44.—Sacral hump barely noticeable; tail reduced to a stub; corner
of mouth at level of pupil of eye; dorsal surfaces pale olive-green; venter
white.
Changes proceed in a definite pattern during the growth and development
of tadpoles. Larval teeth are absent in hatchlings; the inner tooth-rows develop
first, and the third lower row last. At metamorphosis the third lower
row is the first to be lost. The tail increases gradually in length relative to
the body. In stage 25 the tail is 52.1 per cent of the total length, and in stage
36, 64.6 per cent. In later stages the tail becomes relatively shorter through
resorption. Duellman and Klaas (1964:320) noted a great size-variation in
Triprion tadpoles in stage 25. No such variation is apparent in any stage of
any of the species of Smilisca studied.
The growth and development of the other species of Smilisca do not differ
significantly from that of S. phaeota. The tadpoles of S. sila and sordida from
streams have relatively longer tails at hatching. For example, in tadpoles of
S. sordida the average length of tail is 64.0 per cent of the body-length in stage
25, and in stage 37, 67.0 per cent.
Behavior
The tadpoles of S. baudini, cyanosticta, phaeota, and puma are pelagic inhabitants
of shallow ponds. Early stages of S. baudini in which external gills
[Pg 366]
are present have been observed to hang vertically with the gills spread out at
the surface of the water, a behavior noted by Zweifel (1964:206) in tadpoles
of Phrynohyas venulosa, which also develop in warm, standing water having a
relatively low oxygen-tension. When disturbed the pelagic tadpoles usually
dive and seek shelter amidst vegetation or in mud on the bottom. This behavior
was observed in S. baudini, cyanosticta, and phaeota by day and at
night. No tadpoles of S. puma were observed by day; those seen at night
were near the surface of small water-filled depressions in a grassy marsh; they
responded to light by taking refuge in the dense grass. Perhaps tadpoles of
this species are negatively phototactic and remain hidden by day.
The stream-inhabiting tadpoles of S. sila and sordida live in clear pools in
rocky streams, where they were observed to cling by their mouths to rocks in
the stream and to seek shelter amidst pebbles or beneath rocks and leaves on
the bottom. These tadpoles are not found in shallow riffles.
We have not found tadpoles of two species of Smilisca in the same body
of water and therefore cannot offer observations on ecological relationships in
sympatric situations.
Identifiable hylid remains are known from the Miocene to the Recent, but
these fossils are mostly fragmentary and provide little useful information regarding
the phylogenetic relationships of living genera. Frogs of the genus
Smilisca are generalized and show no striking adaptations, either in their structure
or in their modes of life history.
Interspecific Relationships
In attempting to understand the relationships of the species of Smilisca we
have emphasized osteological characters. The phylogeny suggested by these
characters is supported by other lines of evidence, including external morphology,
tadpoles, and breeding calls.
Our concept of the prototype of the genus Smilisca is a moderate-sized
hylid having: (1) a well-developed frontoparietal fontanelle, (2) frontoparietal
lacking lateral processes, (3) no bony squamosal-maxillary arch, (4) a fully
ossified ethmoid, (5) paired subgular vocal sac, (6) moderately webbed
fingers and toes, (7) relatively few supernumerary tubercles on the digits, (8)
eggs deposited in clumps in ponds, (9) anteroventral mouth in tadpoles
bordered by one row of labial papillae, but median part of upper lip bare,
(10) tail relatively short and deep in tadpoles, and (11) a breeding call consisting
of a series of like notes.
Two phyletic lines evolved from this prototype. The first of these was the
stock that gave rise to the baudini group. The evolutionary changes that
took place in this line included increase in size, development of a lateral
curvature of the maxillary, and an increased amount of cranial ossification,
especially in the dermal roofing bones. This phyletic line retained the larval
characters and breeding call of the prototype. The second phyletic line gave
rise to the sordida group and diverged from the prototype in the development
of an angular maxillary and a breeding call consisting of a primary note followed
by secondary notes. The frogs in this phyletic line retained the moderate
[Pg 367]
size of the prototype and did not develop additional dermal bone. Our concept
of the phylogenetic relationships is shown graphically in Figure 17.
Within the baudini group one stock retained separate nasals and did not
develop a bony squamosal-maxillary arch, but broad lateral processes developed
on the frontoparietals. The tadpoles remained unchanged from the primitive
type. This stock evolved into S. phaeota. In the other stock the nasals became
fully ossified and a bony squamosal-maxillary arch developed. One
branch of this second stock retained tadpoles having only one row of labial
papillae and did not develop lateral processes on the frontoparietals; this
branch evolved into S. cyanosticta. The other branch diverged and gave rise
to S. baudini by developing relatively shorter hind legs, large lateral processes
on the frontoparietals, and tadpoles having two rows of labial papillae.
Within the sordida group the cranial features remained unchanged in one
line, which gave rise to S. sila, whereas in a second line the nasals were reduced,
and their long axes shifted with the result that they are not parallel to
the maxillaries; the amount of ossification of the ethmoid was reduced, and
the tadpoles developed two rows of labial papillae. In this second line one
branch retained the pond-breeding habits and gave rise to S. puma, whereas
a second branch became adapted to stream-breeding and gave rise to S.
sordida.
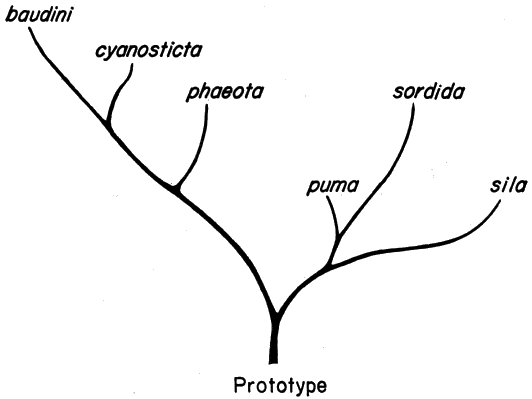
phylogenetic relationships of the species of Smilisca.
Certain aspects of this proposed phylogeny warrant further comment.
Features such as the deposition of additional bone that roofs the skull or
that forms lateral projections from the frontoparietals, like those in S. baudini
and phaeota, are minor alterations of dermal elements and not basic modifications
[Pg 368]
of the architecture of the skull. Consequently, we hypothesize the independent
development of these dermal changes in S. baudini and phaeota.
Similar kinds of dermal modifications have evolved independently in many
diverse groups of frogs.
Likewise, we propose the parallel development of stream-adapted tadpoles
in S. sordida and sila; in both cases the tadpoles adapted to changing environmental
conditions (see following section on evolutionary history). Tadpoles of
S. sordida already had two rows of labial papillae before entering the streams;
subsequently the tadpoles developed complete rows of papillae, ventral mouths
and long tails having low fins. Possibly the tadpoles of S. sila had two rows
of labial papillae prior to their adapting to stream conditions; in the process
of adapting they developed ventral mouths and long tails having low fins.
Similar modifications in tadpoles have occurred in many diverse groups of
Middle American hylids, such as Plectrohyla, Ptychohyla, the Hyla uranochroa
group, and the Hyla taeniopus group.
Our lack of concern about coloration is due to the fact that, with the exception
of the blue spots on the flanks and posterior surfaces of the thighs in some
species, the coloration of Smilisca, consisting of a pattern of irregular dark
marks on a paler dorsum and dark transverse bars on the limbs, is not much
different from that of many other Neotropical hylids. Blue is a structural color,
rare among Amphibia, which is achieved by the absence of lipophores above
the guanophores. Thus, the incident light rays at the blue end of the spectrum
are reflected by the guanophores without interference by an overlying yellow
lipophore screen. According to Noble (1931), lipophores are capable of
amoeboid movement that permits shifts in their positions, between or beneath
the guanophores. We do not know whether this behavior of lipophores is
widespread and is effected in response to environmental changes, or whether
it is a genetically controlled attribute that is restricted in appearance. If the
latter is the case we must assume that the prototype of Smilisca possessed such
an attribute which was lost in S. baudini, phaeota, and puma. The development
of blue spots is not constant in S. sordida and S. sila; in S. cyanosticta
the spots range in color from blue to pale green.
The coloration of the tadpoles is not distinctive, except for the presence of
dorsal blotches on the tails of S. sila and sordida. However, the similarity in
pattern cannot be interpreted as indicating close relationships because nearly
identical patterns are present in Hyla legleri and some species of Prostherapis.
This disruptive coloration seems to be directly associated with the pebble-bottom,
stream-inhabiting tadpoles.
In the baudini group, S. phaeota and cyanosticta are allopatric, whereas S.
baudini occurs sympatrically with both of those species. The call of S. baudini
differs notably from the calls of S. phaeota and cyanosticta, which are more
nearly alike. Although in the phylogenetic scheme proposed here S. sila is
considered to be more distantly related to S. puma than is S. sordida, the calls
of S. sila and puma more closely resemble one another than either resembles
that of S. sordida. Smilisca sila and puma are allopatric, whereas S. sordida
is broadly sympatric with both of those species. We assume that in their
respective phyletic lines the differentiation of both S. baudini and sordida was
the result of genetic changes in geographically isolated populations. Subsequently,
each species dispersed into areas inhabited by other members of their
respective groups. Selection for differences in the breeding calls helped to
[Pg 369]
reinforce other differences in the populations and thereby aided in maintaining
specificity.
Evolutionary History
With respect to temporal and spatial aspects of evolution in Smilisca, we
have tried to correlate the phylogenetic evidence on Smilisca with the geologic
data on Middle America presented by Lloyd (1963), Vinson and Brineman
(1963), Guzmán and Cserna (1963), Maldonado-Koerdell (1964), and Whitmore
and Stewart (1965). Likewise, we have borne in mind the evidence for,
and ideas about, the evolution of the Middle American herpetofauna given by
Dunn (1931b), Schmidt (1943), Stuart (1950, 1964) Duellman (1958, MS),
and Savage (MS).
According to Stuart's (1950) historical arrangement of the herpetofauna,
Smilisca is a member of the Autochthonous Middle American Faunal Element,
and according to Savage's (MS) arrangement the genus belongs to the Middle
American Element, a fauna which was derived from a generalized tropical
American unit that was isolated in tropical North America by the inundation
of the Isthmian Link in early Tertiary, that developed in situ in tropical North
America, and that was restricted to Middle America by climatic change in the
late Cenozoic.
Savage (MS) relied on the paleogeographic maps of Lloyd (1963) to
hypothesize the extent and centers of differentiation of the Middle American
Faunal Element. According to Lloyd's concept, Middle America in the Miocene
consisted of a broad peninsula extending southeastward to about central
Nicaragua, separated from the Panamanian Spur of continental South America
by shallow seas. A large island, the Talamanca Range, and remnants of the
Guanarivas Ridge formed an archipelago in the shallow sea. The recent discovery
of remains of mammals having definite North American affinities in the
Miocene of the Canal Zone (Whitmore and Stewart, 1965) provides substantial
evidence that at least a peninsula was continuous southeastward from Nuclear
Central America to the area of the present Canal Zone in early mid-Miocene
time. South America was isolated from Central America by the Bolivar Trough
until late mid-Pliocene.
Thus, in the mid-Tertiary the broad peninsula of Nuclear Central America,
which consisted of low and moderately uplifted regions having a tropical
mesic climate, provided the site for the evolution of Smilisca. It is not possible
to determine when the genus evolved, but to explain the differentiation of
the species it is unnecessary to have the ancestral Smilisca present prior to the
Miocene.
We view the Miocene Smilisca as the prototype described in the preceding
section, and suppose that it lived in the mesic tropical environment of the
eastern part of the Central American Peninsula (in what is now Costa Rica
and western Panamá). Two stocks differentiated, probably in middle Miocene
times; one of these, the ancestral stock of the baudini group, was widespread
on the Caribbean lowlands from the Nicaraguan Depression to the Bolivar
Trough, and the other, the ancestral stock of the sordida group, was restricted
to the Pacific lowlands of the same region. In late Miocene time the ancestral
stock of the baudini group dispersed northwestward around the deep embayment
in the Nicaraguan depression into upper Central America (in what is
now Honduras and Guatemala) and thence into southern México. Apparently
[Pg 370]
differentiation took place on each side of the Nicaraguan Depression; the frogs
to the south of the depression evolved into S. phaeota, whereas those to the
north of the depression represented the stock from which S. baudini and
cyanosticta arose. Prior to the uplift of the mountains in the late Miocene
and the Pliocene the baudini-cyanosticta stock probably was widespread in
northwestern Central America. The elevation of the mountains resulted in
notable climatic changes, principally the development of sub-humid environments
on the Pacific lowlands. The frogs living on the Pacific lowlands became
adapted to sub-humid conditions and developed into S. baudini. The
stock on the Caribbean lowlands remained in mesic environments and evolved
into S. cyanosticta.
Possibly in the middle Miocene before the Talamanca Range in Costa Rica
and western Panamá was greatly uplifted, the ancestral stock of the sordida
group invaded the Caribbean lowlands of what is now Costa Rica. The subsequent
elevation of the Talamanca Range in the Pliocene effectively isolated
the ancestral stock of S. sila on the Pacific lowlands from the puma-sordida
stock on the Caribbean lowlands. The former was subjected to the sub-humid
conditions which developed on the Pacific lowlands when the Talamanca
Range was uplifted. It adapted to the sub-humid environment by living
along streams and evolving stream-adapted tadpoles. On the Caribbean side
of the Talamanca Range the puma-sordida stock inhabited mesic environments.
The stock that evolved into S. puma remained in the lowlands as a pond-breeding
frog, whereas those frogs living on the slopes of the newly elevated
mountains became adapted for their montane existence by developing stream-adapted
tadpoles and thus differentiated into S. sordida.
Probably the six species of Smilisca were in existence by the end of the
Pliocene; at that time a continuous land connection existed from Central America
to South America. The climatic fluctuations in the Pleistocene, and the
post-Wisconsin development of present climatic and vegetational patterns in
Middle America, brought about the present patterns of distribution of the
species. From its place of origin on the Caribbean lowlands of lower Central
America, S. phaeota dispersed northward into Nicaragua and southward along
the Pacific slopes of northwestern South America. Perhaps in the late Pleistocene
or in post-Wisconsin time when mesic conditions were more widespread
than now, S. phaeota moved onto the Pacific lowlands of Costa Rica. Its route
could have been through the Arenal Depression. Subsequent aridity restricted
its range on the Pacific lowlands to the Golfo Dulce region. Climatic fluctuation
in northern Central America restricted the distribution of S. cyanosticta
to mesic habitats on the slopes of the Mexican and Guatemalan highlands and
to certain humid areas on the lowlands. Smilisca baudini was well adapted
to sub-humid conditions, and the species dispersed northward to the Rio
Grande Embayment and to the edge of the Sonoran Desert and southward into
Costa Rica. In southern México and Central America the species invaded
mesic habitats. Consequently, in some areas it is sympatric with S. cyanosticta
and phaeota.
Smilisca puma dispersed northward onto the Caribbean lowlands of southern
Nicaragua. Its southward movements probably were limited by the ridges of
the Talamanca Range that extend to the Caribbean coast in the area of Punta
Cahuita in Costa Rica. Smilisca sila dispersed along the Pacific lowlands and
slopes of the mountains from eastern Costa Rica and western Panamá through
[Pg 371]
eastern Panamá to northern Colombia. Climatic fluctuation in the Pleistocene
evidently provided sufficient altitudinal shifts in environments in the Talamanca
Range to permit S. sordida to move onto the Pacific slopes. From its upland
distribution the species followed streams down to both the Caribbean and
Pacific lowlands, where it is sympatric with S. puma on the Caribbean lowlands
and S. sila on the Pacific lowlands.
The evolution of the species-groups of Smilisca was effected through isolation
by physical barriers in the Cenozoic; the differentiation of the species was
initiated by further isolation of populations by changes in physiography and
climate. Present patterns of distribution resulted from Pleistocene and post-Wisconsin
climatic changes. Today, sympatric species have different breeding
habits and breeding calls which reinforce the differences in morphology.
SUMMARY AND CONCLUSIONS
The genus Smilisca is composed of six species of tree frogs; each species is
defined on the basis of adult morphology, larval characters, and breeding behavior.
Keys are provided to aid in the identification of adults and of tadpoles.
Analysis of the characters and examination of type specimens indicates that
several currently-recognized taxa are synonymous, as follows:
1. Hyla beltrani Taylor, 1942 = Smilisca baudini.
2. Hyla gabbi Cope, 1876 = Smilisca sordida.
3. Hyla manisorum Taylor, 1954 = Smilisca baudini.
4. Hyla nigripes Cope, 1876 = Smilisca sordida.
5. Hyla wellmanorum Taylor, 1952 = Smilisca puma.
Smilisca phaeota cyanosticta Smith, 1953 is elevated to specific rank, and
one new species, Smilisca sila, is named and described.
The skeletal system of developmental stages and the adult of Smilisca baudini
is described, and the skull is compared with that of other members of the
genus.
The tadpoles are described, compared, and illustrated; the larval development
of Smilisca phaeota is described.
Breeding behavior and breeding calls are described and compared. Some
species of Smilisca have breeding choruses. Two species, S. sila and sordida,
breed in streams, whereas the others breed in ponds.
The genus is considered to be part of the Middle American Faunal Element;
the species are thought to have differentiated in response to ecological diversity
and historical opportunities provided by Cenozoic changes in physiography and
climate.
[Pg 372]
LITERATURE CITED
Proc. Acad. Nat. Sci. Philadelphia, 7:59-62. April 27.
survey. Washington, D. C., p. 35, pl. 41.
Jour. Morph., 104:527-560. May.
Mus. Zool. Univ. Michigan, 129:1-16. January 25.
Nat., 3:77-89. June 1, 1959.
June.
Bufo: A progress report. Evolution, 17:1-16. March.
in Lanyon, W. E. and Tavolga, W. N. (Eds.) Animal sounds and
communication, pp. 137-320.
Zool., 6:70-74. June.
Panama, with notes on their life histories and habits. Bull. Amer.
Mus. Nat. Hist., 86:375-436, pls. 42-60. August 26.
au Guatemala. Bull. Soc. Philom. Paris, ser. 7, 1:122-132.
Occas. Papers Mus. Zool. Univ. Michigan, 555:1-19, pl. 1. July 16.
National Museum. Bull. U. S. Nat. Mus., 220:xv + 291 pp.
Parana, Paraguay, Vermejo and Uraguay rivers.... Proc.
Acad. Nat. Sci. Philadelphia, 14, pt. 9:346-359.
Acad. Nat. Sci. Philadelphia, 17:185-198. October.
Acad. Nat. Sci. Philadelphia, 23, pt. 2:200-224.
South Wales, 82, pt. 1:9-108. September.
Publ. Mus. Zool. Univ. Michigan, 96:1-47, pls. 1-6. February 21.
Amer. Mus. Nat. Hist., 114:1-152, pls. 1-31. February 24.
[Pg 373]
Univ. Kansas Publ. Mus. Nat. Hist., 15:297-349, pls. 11-18. October
18.
Rev. Biol. Trop., 11(1):1-23. October.
Publ. Mus. Nat. Hist., 15:469-491. March 2.
no. 2:159-168. June 25.
June 30.
vol. 8, 792 pp.
Soc. Nat. Hist., 5:403-421. October 10.
October 30.
Exact., Fis. Nat., 6:68-81.
Zone. Evolution, 14:484-497. December.
Occas. Papers Nat. Hist. Mus. Stanford Univ., 5:1-89. April 1.
pp.
Nicaragua. Occas. Papers Mus. Zool. Univ. Michigan, 357:1-18.
October 26.
36:5-18. July 14.
notes on identification. Herpetologica, 16:183-190. September 23.
Brachycephalidae (Amphibia, Salientia). Proc. Zool. Soc. London,
132:457-487, pls. 1-4.
2:113-129.
Zool., 11:39-44. March.
Zool., 12:20-35. March.
Conte in Texas. Copeia, no. 4:327-335. December 30.
[Pg 374]
Assoc. Petrol. Geol., Mem. 2:88-100.
R. and West, R. C. (Eds.). Handbook of Middle American Indians,
vol. 1, Univ. Texas Press, Austin, 570 pp.
19:122-128. July 3.
2:80-83. May 29.
Syst. Zool., 6:79-86. June.
Berlin, pp. 445-471.
andere neue oder weniger bekannte Amphibien. Monats. Konigl.
Akad. Wiss. Berlin, pp. 603-618, pl. 5. October 16.
genus Pseudis. Zoologica, 38:193-200.
Mus. Nat. Hist., 22:475-510. December 30.
Midl. Nat., 30:241-253. July.
Sitzungb. Konigl. Akad. Wiss. Math.-Natur. Cl., 24(1):10-15.
March.
K. K. Akad. Wiss. Math.-Natur. Cl., 14(2):237-258, pls. 1-3.
America. Herpetologica, 8:150-152. January 30.
Sci. Bull., 33:313-380. March 20.
30.
California Publ. Zool., 56:497-540. February 17.
nigrita triseriata. Copeia, no. 3:211-217. July 29.
[Pg 375]
the savanna region of central Petén, Guatemala. Misc. Publ. Mus.
Zool. Univ. Michigan, 29:1-56, pls. 1-4, 1 map. October 1.
Publ. Mus. Zool. Univ. Michigan, 69:1-109. June 12.
Contr. Lab. Vert. Biol., 45:1-77, pls. 1-9, 1 map. May.
Lab. Vert. Biol., 68:1-65, pls. 1-4. November.
El Petén, Guatemala. Contr. Lab. Vert. Biol., 75:1-30. June.
dorsalis Duméril and Bibron. Herpetologica, 17:73-79.
July 11.
(Eds.). Handbook of Middle American Indians, vol. 1, Univ.
Texas Press, Austin, 570 pp.
28:295-323. November 15.
July 1.
comments on other species. No. I. Univ. Kansas Sci. Bull.,
36:597-639. June 1.
the Walter Rathbone Bacon Traveling Scholarship. Proc. U. S.
Natl. Mus., 95:521-613, pls. 18-32. June 30.
67:157-183. January.
Assoc. Petrol. Geol., Mem. 2:101-112.
April 9.
relationships to fossil and Recent forms. Amer. Mus. Novitates,
1762:1-45. April 6.
hylid genus Nyctimystes. Amer. Mus. Novitates, 1896:1-51. July
22.
Panamá. Copeia, no. 1:201-208. March 26.
Transmitted March 14, 1966.
31-3430
[Pg i]
MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this
series by addressing the Exchange Librarian, University of Kansas Library,
Lawrence, Kansas. Copies for individuals, persons working in a particular
field of study, may be obtained by addressing instead the Museum of Natural
History, University of Kansas, Lawrence, Kansas. When individuals request
copies from the Museum, 25 cents should be included, for each 100 pages or
part thereof, for the purpose of defraying the costs of wrapping and mailing.
For certain longer papers an additional amount, indicated below, toward some
of the costs of production, is to be included.
* An asterisk designates those numbers of which the Museum's supply is exhausted.
| Vol. 1. | Nos. 1-26 and index. Pp. 1-638, 1946-1950. | |
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
| *4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | Nos. 1-37 and index. Pp. 1-676, 1951-1953. | |
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | Nos. 1-15 and index. Pp. 1-651, 1952-1955. | |
| Vol. 8. | Nos. 1-10 and index. Pp. 1-675, 1954-1956. | |
| Vol. 9. | Nos. 1-23 and index. Pp. 1-690, 1955-1960. | |
| Vol. 10. | Nos. 1-10 and index. Pp. 1-626, 1956-1960. | |
| Vol. 11. | Nos. 1-10 and index. Pp. 1-703, 1958-1960. | |
| Vol. 12. | *1. | Functional morphology of three bats: Eumops, Myotis, Macrotus. By Terry A. Vaughan. Pp. 1-153, pls. 1-4, 24 figures in text. July 8, 1959. |
| *2. | The ancestry of modern Amphibia: a review of the evidence. By Theodore H. Eaton, Jr. Pp. 155-180, 10 figures in text. July 10, 1959. | |
| 3. | The baculum in microtine rodents. By Sidney Anderson. Pp. 181-216, 49 figures in text. February 19, 1960. | |
| *4. | A new order of fishlike Amphibia from the Pennsylvanian of Kansas. By Theodore H. Eaton, Jr., and Peggy Lou Stewart. Pp. 217-240, 12 figures in text. May 2, 1960. | |
| 5. | Natural history of the Bell Vireo, Vireo bellii Audubon. By Jon C. Barlow. Pp. 241-296, 6 figures in text. March 7, 1962. | |
| 6. | Two new pelycosaurs from the lower Permian of Oklahoma. By Richard C. Fox. Pp. 297-307, 6 figures in text. May 21, 1962. | |
| 7. | Vertebrates from the barrier island of Tamaulipas, México. By Robert K. Selander, Richard F. Johnston, B. J. Wilks, and Gerald G. Raun. Pp. 309-345, pls. 5-8. June 18, 1962. | |
| 8. | Teeth of edestid sharks. By Theodore H. Eaton, Jr. Pp. 347-362, 10 figures in text. October 1, 1962. | |
| 9. | Variation in the muscles and nerves of the leg in two genera of grouse (Tympanuchus and Pedioecetes). By E. Bruce Holmes. Pp. 363-474, 20 figures in text. October 25, 1963. $1.00. | |
| 10. | A new genus of Pennsylvanian fish (Crossopterygii, Coelacanthiformes) from Kansas. By Joan Echols. Pp. 475-501, 7 figures in text. October 25, 1963. | |
| 11. | Observations on the Mississippi kite in southwestern Kansas. By Henry S. Fitch. Pp. 503-519. October 25, 1963. | |
| 12. | Jaw musculature of the Mourning and White-winged doves. By Robert L. Merz. Pp. 521-551, 22 figures in text. October 25, 1963. | |
| 13. | Thoracic and coracoid arteries in two families of birds, Columbidae and Hirundinidae. By Marion Anne Jenkinson. Pp. 553-573, 7 figures in text. March 2, 1964. | |
| 14. | The breeding birds of Kansas. By Richard F. Johnston. Pp. 575-655, 10 figures in text. May 18, 1964. 75 cents. | |
| 15. | The adductor muscles of the jaw in some primitive reptiles. By Richard C. Fox. Pp. 657-680, 11 figures in text. May 18, 1964. | |
| Index. Pp. 681-694. | ||
| Vol. 13.[Pg ii] | 1. | Five natural hybrid combinations in minnows (Cyprinidae). By Frank B. Cross and W. L. Minckley. Pp. 1-18. June 1, 1960. |
| 2. | A distributional study of the amphibians of the Isthmus of Tehuantepec, México. By William E. Duellman. Pp. 19-72, pls. 1-8, 3 figures in text. August 16, 1960. 50 cents. | |
| 3. | A new subspecies of the slider turtle (Pseudemys scripta) from Coahulia, México. By John M. Legler. Pp. 73-84, pls. 9-12, 3 figures in text. August 16, 1960. | |
| *4. | Autecology of the copperhead. By Henry S. Fitch. Pp. 85-288, pls. 13-20, 26 figures in text. November 30, 1960. | |
| 5. | Occurrence of the garter snake, Thamnophis sirtalis, in the Great Plains and Rocky Mountains. By Henry S. Fitch and T. Paul Maslin. Pp. 289-308, 4 figures in text. February 10, 1961. | |
| 6. | Fishes of the Wakarusa river in Kansas. By James E. Deacon and Artie L. Metcalf. Pp. 309-322, 1 figure in text. February 10, 1961. | |
| 7. | Geographic variation in the North American cyprinid fish, Hybopsis gracilis. By Leonard J. Olund and Frank B. Cross. Pp. 323-348, pls. 21-24, 2 figures in text. February 10, 1961. | |
| 8. | Descriptions of two species of frogs, genus Ptychohyla; studies of American hylid frogs, V. By William E. Duellman. Pp. 349-357, pl. 25, 2 figures in text. April 27, 1961. | |
| 9. | Fish populations, following a drought, in the Neosho and Marais des Cygnes rivers of Kansas. By James Everett Deacon. Pp. 359-427, pls. 26-30, 3 figures in text. August 11, 1961. 75 cents. | |
| 10. | Recent soft-shelled turtles of North America (family Trionychidae). By Robert G. Webb. Pp. 429-611, pls. 31-54, 24 figures in text, February 16, 1962. $2.00. | |
| Index. Pp. 613-624. | ||
| Vol. 14. | 1. | Neotropical bats from western México. By Sydney Anderson. Pp. 1-8. October 24, 1960. |
| 2. | Geographic variation in the harvest mouse. Reithrodontomys megalotis, on the central Great Plains and in adjacent regions. By J. Knox Jones, Jr., and B. Mursaloglu. Pp. 9-27, 1 figure in text. July 24, 1961. | |
| 3. | Mammals of Mesa Verde National Park, Colorado. By Sydney Anderson. Pp. 29-67, pls. 1 and 2, 3 figures in text. July 24, 1961. | |
| 4. | A new subspecies of the black myotis (bat) from eastern Mexico. By E. Raymond Hall and Ticul Alvarez. Pp. 69-72, 1 figure in text. December 29, 1961. | |
| 5. | North American yellow bats, "Dasypterus," and a list of the named kinds of the genus Lasiurus Gray. By E. Raymond Hall and J. Knox Jones, Jr. Pp. 73-98, 4 figures in text. December 29, 1961. | |
| 6. | Natural history of the brush mouse (Peromyscus boylii) in Kansas with description of a new subspecies. By Charles A. Long. Pp. 99-110, 1 figure in text. December 29, 1961. | |
| 7. | Taxonomic status of some mice of the Peromyscus boylii group in eastern Mexico, with description of a new subspecies. By Ticul Alvarez. Pp. 111-120, 1 figure in text. December 29, 1961. | |
| 8. | A new subspecies of ground squirrel (Spermophilus spilosoma) from Tamaulipas, Mexico. By Ticul Alvarez. Pp. 121-124. March 7, 1962. | |
| 9. | Taxonomic status of the free-tailed bat, Tadarida yucatanica Miller. By J. Knox Jones, Jr., and Ticul Alvarez. Pp. 125-133, 1 figure in text. March 7, 1962. | |
| 10. | A new doglike carnivore, genus Cynaretus, from the Clarendonian Pliocene, of Texas. By E. Raymond Hall and Walter W. Dalquest. Pp. 135-138, 2 figures in text. April 30, 1962. | |
| 11. | A new subspecies of wood rat (Neotoma) from northeastern Mexico. By Ticul Alvarez. Pp. 139-143. April 30, 1962. | |
| 12. | Noteworthy mammals from Sinaloa, Mexico. By J. Knox Jones, Jr., Ticul Alvarez, and M. Raymond Lee. Pp. 145-159, 1 figure in text. May 18, 1962. | |
| 13. | A new bat (Myotis) from Mexico. By E. Raymond Hall. Pp. 161-164, 1 figure in text. May 21, 1962. | |
| *14. | The mammals of Veracruz. By E. Raymond Hall and Walter W. Dalquest. Pp. 165-362, 2 figures. May 20, 1963. $2.00. | |
| 15. | The recent mammals of Tamaulipas, México. By Ticul Alvarez. Pp. 363-473, 5 figures in text. May 20, 1963. $1.00. | |
| 16. | A new subspecies of the fruit-eating bat, Sturnira ludovici, from western Mexico. By J. Knox Jones, Jr., and Gary L. Phillips. Pp. 475-481, 1 figure in text. March 2, 1964. | |
| 17. | Records of the fossil mammal Sinclairella, Family Apatemyidae, from the Chadronian and Orellan. By William A. Clemens, Jr. Pp. 483-491, 2 figures in text. March 2, 1964. | |
| 18. | The mammals of Wyoming. By Charles A. Long. Pp. 493-758, 82 figures in text. July 6, 1965. $3.00. | |
| Index. Pp. 759-784. | ||
| Vol. 15. [Pg iii] | 1. | The amphibians and reptiles of Michoacán, México. By William E. Duellman. Pp. 1-148, pls. 1-6, 11 figures in text. December 20, 1961. $1.50. |
| 2. | Some reptiles and amphibians from Korea. By Robert G. Webb, J. Knox Jones, Jr., and George W. Byers. Pp. 149-173. January 31, 1962. | |
| 3. | A new species of frog (Genus Tomodactylus) from western México. By Robert G. Webb. Pp. 175-181, 1 figure in text. March 7, 1962. | |
| 4. | Type specimens of amphibians and reptiles in the Museum of Natural History, the University of Kansas. By William E. Duellman and Barbara Berg. Pp. 183-204. October 26, 1962. | |
| 5. | Amphibians and Reptiles of the Rainforests of Southern El Petén, Guatemala. By William E. Duellman. Pp. 205-249, pls. 7-10, 6 figures in text. October 4, 1963. | |
| 6. | A revision of snakes of the genus Conophis (Family Colubridae, from Middle America). By John Wellman. Pp. 251-295, 9 figures in text. October 4, 1963. | |
| 7. | A review of the Middle American tree frogs of the genus Ptychohyla. By William E. Duellman. Pp. 297-349, pls. 11-18, 7 figures in text. October 18, 1963. 50 cents. | |
| 8. | Natural history of the racer Coluber constrictor. By Henry S. Fitch. Pp. 351-468, pls. 19-22, 20 figures in text. December 30, 1963. $1.00. | |
| 9. | A review of the frogs of the Hyla bistincta group. By William E. Duellman. Pp. 469-491, 4 figures in text. March 2, 1964. | |
| 10. | An ecological study of the garter snake, Thamnophis sirtalis. By Henry S. Fitch. Pp. 493-564, pls. 23-25, 14 figures in text. May 17, 1965. | |
| 11. | Breeding cycle in the ground skink, Lygosoma laterale. By Henry S. Fitch and Harry W. Greene. Pp. 565-575, 3 figures in text. May 17, 1965. | |
| 12. | Amphibians and reptiles from the Yucatan Peninsula, México. By William E. Duellman. Pp. 577-614, 1 figure in text. June 22, 1965. | |
| 13. | A new species of turtle, genus Kinosternon, from Central America. By John M. Legler. Pp. 615-625, pls. 26-28, 2 figures in text. June 20, 1965. | |
| 14. | A biogeographic account of the herpetofauna of Michoacán, México. By William E. Duellman. Pp. 627-709, pls. 29-36, 5 figures in text. December 30, 1965. | |
| 15. | Amphibians and reptiles of Mesa Verde National Park, Colorado. By Charles L. Douglas. Pp. 711-744, pls. 37 and 38, 6 figures in text. March 7, 1966. | |
| Index in preparation. | ||
| Vol. 16. | 1. | Distribution and taxonomy of mammals of Nebraska. By J. Knox Jones, Jr. Pp. 1-356, plates 1-4, 82 figures in text. October 1, 1964. $3.50. |
| 2. | Synopsis of the lagomorphs and rodents of Korea. By J. Knox Jones, Jr., and David H. Johnson. Pp. 357-407. February 12, 1965. | |
| 3. | Mammals from Isla Cozumel, Mexico, with description of a new species of harvest mouse. By J. Knox Jones, Jr. and Timothy E. Lawlor. Pp. 409-419, 1 figure in text. April 13, 1965. | |
| 4. | The Yucatan deer mouse, Peromyscus yucatanicus. By Timothy E. Lawlor. Pp. 421-438, 2 figures in text. July 20, 1965. | |
| 5. | Bats from Gautemala. By J. Knox Jones, Jr. Pp. 439-472. April 18, 1966. | |
| More numbers will appear in volume 16. | ||
| Vol. 17. | 1. | Localities of fossil vertebrates obtained from the Niobrara Formation (Cretaceous) of Kansas. By David Bardack. Pp. 1-14. January 22, 1965. |
| 2. | Chorda tympani branch of the facial nerve in the middle ear of tetrapods. By Richard C. Fox. Pp. 15-21. June 22, 1965. | |
| 3. | Fishes of the Kansas River System in relation to zoogeography of the Great Plains. By Artie L. Metcalf. Pp. 23-189, 4 figures in text, 51 maps. March 24, 1966. | |
| 4. | Factors affecting growth and production of channel catfish, Ictalurus punctatus. By Bill A. Simco and Frank B. Cross. Pp. 191-256, 13 figures in text. June 6, 1966. | |
| 5. | A new species of fringe-limbed tree frog, genus Hyla, from Darién, Panamá. By William E. Duellman. Pp. 257-262, 1 figure in text. June 17, 1966. | |
| 6. | Taxonomic notes on some Mexican and Central American hylid frogs. By William E. Duellman. Pp. 263-279. June 17, 1966. | |
| 7. | Neotropical hylid frogs, genus Smilisca. By William E. Duellman and Linda Trueb. Pp. 281-375, pls. 1-12, 17 figures in text. July 14, 1966. | |
| More numbers will appear in volume 17. | ||
With the exception of the corrections listed below and several minor
corrections not listed, the text presented is that which appeared in
the original printed version. The list of Kansas University publications
has been compiled at the end of the article. The cover displayed was
compiled from a image of the original cover with graphics from the
article added.
| Page | Correction | |
| 287 | cleared ⇒ cleaned | |
| 292 | Data based of => Data based on | |
| 298 | CNMH ⇒ CNHM | |
| 299 | Acahuitzotla ⇒ Acahuizotla | |
| 304 | cyanostica ⇒ cyanosticta | |
| 305 | Quatemala ⇒ Guatemala | |
| 307 | cyanostica ⇒ cyanosticta | |
| 313 | Matagalapa ⇒ Matagalpa | |
| 322 | Carribean ⇒ Caribbean | |
| 323 | Centralia ⇒ Centrali | |
| 336 | proportionaely ⇒ proportionately | |
| 346 | noticably ⇒ noticeably | |
| 372 | Carvaljo ⇒ Carvalho | |
| 375 | Dumeril ⇒ Duméril | |
| ii | trutles ⇒ turtles |
Featured Books

Old French Romances, Done into English
William Morris
ty that these cameos ofromance should suffer the same fate as Mr. Lang’s versionof Aucassin et Nic...

The Eagle's Shadow
James Branch Cabell
cation of the Impecunious, Margaret's almoner in furthering thecause of charity and philanthropy. Ka...

The Pigmy Woodrat, Neotoma goldmani, Its Distribution and Systematic Position
Rollin H. Baker and Dennis G. Rainey
te Univ. Studies, Biol. Sci. Ser.No. 1:162, December 28, 1953) extended the known distributionof thi...

Artistic Anatomy of Animals
Édouard Cuyer
of preparation, and we hadcollected materials for it, with the object of filling up avoid of which t...

The People of the Mist
H. Rider Haggard
T ARGUMENT XXXV. BE NOBLE OR BE BASE XXXVI. HOW OTTER CAME BACK XXXVII. “I AM REPAID, QUEEN” XXX...

Dan, the Newsboy
Jr. Horatio Alger
Well207XXX.—How Hartley Got a Clew215XXXI.—Althea's Abduction222XXXII.—Donovan's229XXXIII.—...

The Genial Idiot: His Views and Reviews
John Kendrick Bangs
1.25Enchanted Type-writer. Ill’d. 16mo 1.25Booming of Acre Hill. Illustrated. 16mo ...

...After a Few Words...
Randall Garrett
santguardant or. Behind the standard-bearer, his great war horse movingwith a steady, measured pace,...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Neotropical Hylid Frogs, Genus Smilisca" :