The Project Gutenberg eBook of A Field Study of the Kansas Ant-Eating Frog, Gastrophryne olivacea
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: A Field Study of the Kansas Ant-Eating Frog, Gastrophryne olivacea
Author: Henry S. Fitch
Release date: August 29, 2010 [eBook #33574]
Most recently updated: January 6, 2021
Language: English
Credits: Produced by Simon Gardner, Chris Curnow, Joseph Cooper and
the Online Distributed Proofreading Team at
https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK A FIELD STUDY OF THE KANSAS ANT-EATING FROG, GASTROPHRYNE OLIVACEA ***
Transcriber's Notes
A small number of inconsistencies and typographical errors have been
changed in the text. These are listed at the end of this book.
The Title page and Verso are in error in stating that the pages run 275 to 306. This should read 276-307.
The caption of Figure 5 states that the illustration is "a little less than twice natural size".
This is accurate for the linked image when viewed at approx. 100 dpi with browser display setting 100%.
The thumbnail image is approximately natural size under the same conditions.
Table of Contents:
- Introduction
- Habitat
- Behavior
- Temperature Relationships
- Breeding
- Development of Eggs and Larvae
- Growth
- Color and Pattern
- Movements
- Food Habits
- Predation
- Summary
- Literature Cited
University of Kansas Publications
Museum of Natural History
Volume 8, No. 4, pp. 275-306, 9 figs. in text
February 10, 1956
A Field Study
of the Kansas Ant-Eating Frog,
Gastrophryne olivacea
BY
HENRY S. FITCH
University of Kansas
Lawrence
1956
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard,
Robert W. Wilson
Volume 8, No. 4, pp. 275-306, 9 figs. in text
Published February 10, 1956
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND. JR., STATE PRINTER
TOPEKA, KANSAS
1956
25-7819
[Pg 277]
A FIELD STUDY
OF THE KANSAS ANT-EATING FROG,
GASTROPHRYNE OLIVACEA
By
Henry S. Fitch
INTRODUCTION
The ant-eating frog is one of the smallest species of vertebrates on
the University of Kansas Natural History Reservation, but individually
it is one of the most numerous. The species is important
in the over-all ecology; its biomass often exceeds that of larger species
of vertebrates. Because of secretive and subterranean habits,
however, its abundance and effects on community associates are
largely obscured.
The Reservation, where my field study was made, is the most
northeastern section in Douglas County, Kansas, and is approximately
5½ miles north and 2½ miles east of the University campus
at Lawrence. The locality represents one of the northernmost occurrences
of the species, genus, and family. The family Microhylidae
is a large one, and most of its representatives are specialized
for a subterranean existence and a diet of termites or ants. The
many subfamilies of microhylids all have distributions centering in
the regions bordering the Indian Ocean, from South Africa and
Madagascar to the East Indies, New Guinea, and Australia (Parker,
1934). Only one subfamily, the Microhylinae, is represented in the
New World, where it has some 17 genera (de Carvalho, 1954)
nearly all of which are tropical. G. olivacea, extending north into
extreme southern Nebraska (Loomis, 1945: 211), ranges farther
north than any other American species. In the Old World only
Kaloula borealis has a comparable northward distribution. Occurring
in the vicinity of Peiping (Pope, 1931: 587), it reaches approximately
the same latitude as does Gastrophryne in Nebraska.
The great majority of microhylid genera and species are confined
to the tropics.
Nearly all ant-eating frogs seen on the Reservation have been
caught and examined and individually marked. By November 1,
1954, 1215 individuals had been recorded with a total of 1472 captures.
In the summer of 1950, Richard Freiburg studied this frog
on the Reservation and his findings (1951) led to a better understanding[Pg 278]
of its natural history. The numbers of frogs studied by him
however, were relatively small and the field work was limited to the
one summer. The data now at hand, representing six consecutive
years, 1949 through 1954, serve to supplement those obtained by
Freiburg, corroborating and extending his conclusions in most instances,
and also indicating that certain of his tentative conclusions
need to be revised.
While the present report was in preparation, Anderson (1954)
published an excellent account of the ecology of the eastern species
G. carolinensis in southern Louisiana. Anderson's findings concerning
this closely related species in a much different environment have
been especially valuable as a basis for comparison. The two species
are basically similar in their habits and ecology but many minor
differences are indicated. Some of these differences result from
the differing environments where Anderson's study and my own
were made and others certainly result from innate genetic differences
between the species.
The frog with which this report is concerned is the Microhyla
carolinensis olivacea of the check list (Schmidt, 1953: 77) and recent
authors. De Carvalho (1954: 12) resurrected the generic name,
Gastrophryne, for the American species formerly included in Microhyla,
and presented seemingly valid morphological evidence for
this plausible generic separation.
G. olivacea is obviously closely related to G. carolinensis; the differences
are not greater than those to be expected between well
marked subspecies. Nevertheless, in eastern Oklahoma and eastern
Texas, where the ranges meet, the two kinds have been found to
maintain their distinctness, differing in coloration, behavior, calls,
and time of breeding. Hecht and Matalas (1946: 2) found seeming
intergrades from the area of overlapping in eastern Texas, but
some specimens from this same area were typical of each form.
Their study was limited to preserved material, in which some characters
probably were obscured. More field work throughout the
zone of contact is needed. The evidence of intergradation obtained
so far seems to be somewhat equivocal.
Besides G. olivacea and typical G. carolinensis there are several
named forms in the genus, including some of doubtful status. The
name mazatlanensis has been applied to a southwestern population,
which seems to be a well marked subspecies of olivacea, but as yet
mazatlanensis has been collected at few localities and the evidence
of intergradation is meager. The names areolata and texensis have
been applied to populations in Texas. Hecht and Matalas (1946: 3)[Pg 279]
consider areolata to be a synonym of olivacea, applied to a population
showing intergradation with carolinensis, but Wright and
Wright (1949: 568) consider areolata to be a distinct subspecies.
G. texensis generally has been considered to be a synonym of
olivacea. Other species of the genus include the tropical G. usta,
G. elegans and G. pictiventris.
Of the vernacular names hitherto applied to G. olivacea none
seems appropriate; I propose to call the species the Kansas ant-eating
frog because of its range extending over most of the state,
and because of its specialized food habits. The type locality,
originally stated to be "Kansas and Nebraska" (Hallowell, 1856:
252) has been restricted to Fort Riley, Kansas (Smith and Taylor,
1950: 358). Members of the genus have most often been referred
to as toads rather than frogs because of their more toadlike appearance
and habits. However, this family belongs to the firmisternial
or froglike division of the Salientia and the terms "frog" and "toad,"
originally applied to Rana and Bufo respectively, have been extended
to include assemblages of related genera or families. Members
of the genus and family usually have been called "narrow-mouthed"
toads from the old generic name Engystoma, a synonym
of Gastrophryne. G. olivacea usually has been referred to as the
Texas narrow-mouthed toad, or western narrow-mouthed toad. The
latter name is inappropriate because the geographic range is between
that of a more western representative (mazatlanensis) and a
more eastern one (carolinensis). The names texensis, areolata and
carolinensis have all been applied to populations in Texas, and it is
questionable whether typical olivacea even extends into Texas.
HABITAT
In the northeastern part of Kansas at least, rocky slopes in open
woods seem to provide optimum habitat conditions. This type of
habitat has been described by several earlier workers in this same
area, Dice (1923: 46), Smith (1934: 503) and Freiburg (1951: 375).
Smith (1950: 113) stated that in Kansas this frog is found in wooded
areas, and that rocks are the usual cover, but he mentioned that outside
of Kansas it is often found in mesquite flats that are devoid of
rocks. Freiburg's field work was done almost entirely on the Reservation
and was concentrated in "Skink Woods" and vicinity, where
much of my own field work, both before and afterward, was concentrated.
On the Reservation and in nearby counties of Kansas,
the habitat preferences of the ant-eating frog and the five-lined skink
largely coincide. In an account of the five-lined skink on the Reservation,[Pg 280]
I have described several study areas in some detail (Fitch,
1954: 37-41). It was on these same study areas (Quarry, Skink
Woods, Rat Woods) that most of the frogs were obtained.
Although G. olivacea thrives in an open-woodland habitat in this
part of its range, it seems to be essentially a grassland species, and
it occurs throughout approximately the southern half of the Great
Plains region. Bragg (1943: 76) emphasized that in Oklahoma it
is widely distributed over the state, occupying a variety of habitats,
with little ecological restriction. Bragg noted, however, that the species
is rarely, if ever, found on extensive river flood plains. On various
occasions I have heard Gastrophryne choruses in a slough two miles
south of the Reservation. This slough is in the Kaw River flood
plain and is two miles from the bluffs where the habitat of rocky
wooded slopes begins that has been considered typical of the species
in northeastern Kansas. It seems that the frogs using this
slough are not drawn from the populations living on the bluffs as
Mud Creek, a Kaw River tributary, intervenes. The creek channel
at times of heavy rainfall, carries a torrent of swirling water which
might present a barrier to migrating frogs as they are not strong
swimmers. The frogs could easily find suitable breeding places much
nearer to the bluffs. Those using the slough are almost certainly
permanent inhabitants of the river flood plain. The area in the
neighborhood of the slough, where the frogs probably live, include
fields of alfalfa and other cultivated crops, weedy fallow fields, and
the marshy margins of the slough. In these situations burrows of
rodents, notably those of the pocket gopher (Geomys bursarius),
would provide subterranean shelter for the frogs, which are not
efficient diggers.
The frogs may live in many situations such as this where they
have been overlooked. In the absence of flat rocks providing hiding
places at the soil surface, the frogs would rarely be found by a collector.
The volume and carrying quality of the voice are much
less than in other common anurans. Large breeding choruses might
be overlooked unless the observer happened to come within a few
yards of them. Most of the recorded habitats and localities of occurrence
may be those where the frog happens to be most in evidence
to human observers, rather than those that are limiting to it or
even typical of it.
On September 20, 1954, after heavy rains, juveniles dispersing
from breeding ponds were in a wide variety of situations, including
most of the habitat types represented on the Reservation. Along
a small dry gully in an eroded field formerly cultivated, and reverted[Pg 281]
to tall grass prairie (big bluestem, little bluestem, switch
grass, Indian grass), the frogs were numerous. Many of them were
flushed by my footsteps from cracks in the soil along the gully banks.
In reaching this area the frogs had moved up a wooded slope from
the pond, crossed the limestone outcrop area at the hilltop edge,
and wandered away from the woods and rocks, out into the prairie
habitat. In this prairie habitat there were no rocks providing hiding
places at the soil surface, but burrows of the vole (Microtus ochrogaster)
and other small rodents provided an abundance of subterranean
shelter. In the summer of 1955 the frogs were seen frequently
in this same area, especially when the soil was wet from
recent rain. When the surface of the soil was dry, none could be
found and presumably all stayed in deep cracks and burrows.
Anderson (1954: 17) indicated that G. carolinensis in Louisiana
likewise occurs in diverse habitats, being sufficiently adaptable to
satisfy its basic requirements in various ways.
BEHAVIOR
Ordinarily the ant-eating frog stays beneath the soil surface, in
cracks or holes or beneath rocks. Probably it obtains its food in such
situations, and rarely wanders on the surface. The occasional individuals
found moving about above ground are in most instances
flushed from their shelters by the vibrations of the observer's footsteps.
On numerous occasions I have noticed individuals, startled
by nearby footfalls, dart from cracks or under rocks and scuttle away
in search of other shelter. Such behavior suggests that digging
predators may be important natural enemies. The gait is a combination
of running and short hops that are usually only an inch or
two in length. The flat pointed head seems to be in contact with
the ground or very near to it as the animal moves about rapidly and
erratically. The frog has a proclivity for squeezing into holes and
cracks, or beneath objects on the ground. The burst of activity by
one that is startled lasts for only a few seconds. Then the frog stops
abruptly, usually concealed wholly or in part by some object. Having
stopped it tends to rely on concealment for protection and may
allow close approach before it flushes again.
Less frequently, undisturbed individuals have been seen wandering
on the soil surface. Such wandering occurs chiefly at night.
Diurnal wandering may occur in relatively cool weather when night
temperatures are too low for the frogs to be active. Wandering
above ground is limited to times when the soil and vegetation are
wet, mainly during heavy rains and immediately afterward.[Pg 282]
Pitfalls made from gallon cans buried in the ground with tops
open and flush with the soil surface were installed in 1949 in several
places along hilltop rock outcrops where the frogs were abundant.
The number of frogs caught from day to day under varying weather-conditions
provided evidence as to the factors controlling surface
activity. After nights of unusually heavy rainfall, a dozen frogs, or
even several dozen, might be found in each of the more productive
pitfalls. A few more might be caught on the following night, and
occasional stragglers as long as the soil remained damp with heavy
dew. Activity is greatest on hot summer nights. Below 20° C.
there is little surface activity but individuals that had body temperatures
as low as 16° C. have been found moving about.
Frogs uncovered in their hiding places beneath flat rocks often
remained motionless depending on concealment for protection, but
if further disturbed, they made off with the running and hopping
gait already described. Although they were not swift, they were
elusive because of their sudden changes of direction and the ease
with which they found shelter. When actually grasped, a frog
would struggle only momentarily, then would become limp with
its legs extended. The viscous dermal secretions copiously produced
by a frog being handled made the animal so slippery that after
a few seconds it might slide from the captor's grasp, and always
was quick to escape when such an opportunity was presented.
TEMPERATURE RELATIONSHIPS
Ant-eating frogs are active over a temperature range of at least
16° C. to 37.6° C. They tolerate high temperatures that would be
lethal to many other kinds of amphibians, but are more sensitive
to low temperatures than any of the other local species, and as a
result their seasonal schedule resembles that of the larger lizards
and snakes more than those of other local amphibians. The latter
become active earlier in the spring.
Earliest recorded dates when the frogs were found active in the
course of the present study from 1950 to 1955 were in April every
year; the 20th, 25th, 24th, 2nd, 25th, and 21st. Latest dates when
the frogs were found in the six years of the study were: October 22,
1949; October 13, 1950; October 7, 1951; August 24, 1952; August
18, 1953; and October 27, 1954 (excluding two late stragglers
caught in a pitfall on December 5). Severe drought caused unseasonably
early retirement in 1952 and 1953.
Body temperatures of the frogs were taken with a small mercury
thermometer of the type described by Bogert (1949: 197); the bulb[Pg 283]
was used to force open the mouth and was thrust down the gullet
into the stomach. To prevent conduction of heat from the hand,
the frog was held down through several layers of cloth, at the spot
where it was discovered, until the temperature reading could be
made. This required approximately five seconds.
Fig. 1. Temperatures of ant-eating frogs grouped in one-degree intervals;
upper figure is of frogs found active in the open, and lower is of those found
under shelter. The frogs are active over a temperature range of more than
20 degrees, and show no clear cut preference within this range.
Most of the 79 frogs of which temperatures were measured, were
found under shelter, chiefly beneath flat rocks. The rocks most
utilized were in open situations, exposed to sunshine. Most of the
frogs were in contact with the warmed undersurfaces of such rocks.
Forty-three of the frogs, approximately 54.5 percent, were in the
eight-degree range between 24° and 31° C. Probably the preferred
temperatures lie within this range. The highest body temperature
recorded, 37.6° C., was in a frog which "froze" and remained motionless
in the sunshine for half a minute after the rock sheltering it
was overturned. Probably its temperature was several degrees
lower while it was sheltered by the rock. Other unusually high temperatures[Pg 284]
were recorded in newly metamorphosed frogs found hiding
in piles of decaying vegetation near the edge of the pond, on
hot afternoons of late August. Temperatures ranged from 17.0° to
30.7° in frogs that were found actually moving about. Several with
relatively low temperatures, 22° to 17°, were juveniles travelling in
rain or mist on cool days. These frogs, having relatively low temperature,
were sluggish in their movements, as compared with individuals
at the upper end of the temperature range.
Fig. 2. Body temperatures and nearby air temperatures for frogs found under
natural conditions. Dots represent frogs found under shelter; circles represent
those found in the open.
After the first frost each year the frogs usually could not be found,
either in the open or in their usual hiding places beneath rocks.
They probably had retired to deep subterranean hibernation sites.
The only exception was in 1954, when two immature frogs were
found together in a pitfall on the morning of December 5 after a[Pg 285]
rain of .55 inches ending many weeks of drought. Air temperature
had been little above 10° C. that night, but had often been below
freezing in the preceding five weeks.
Reactions of these same two individuals to low temperatures were
tested in the laboratory. At a body temperature of 11° C. they were
extremely sluggish. They were capable of slow, waddling movements,
but were reluctant to move and tended to crouch motionless.
Even when they were prodded, they usually did not move away, but
merely flinched slightly. At 6° C. they were even more sluggish,
and seemed incapable of locomotion, as they could not be induced
to hop or walk by prodding with a fine wire. When placed upside
down on a flat surface, they could turn over, but did so slowly,
sometimes only after a minute or more had elapsed. Respiratory
throat movements numbered 46 and 60 per minute.
BREEDING
Many observers have noted that breeding activity is initiated by
heavy rains in summer. In my experience precipitation of at least
two inches within a few days is necessary to bring forth large breeding
choruses. With smaller amounts of precipitation only stragglers
or small aggregations are present at the breeding ponds. Tanner
(1950: 48) stated that in three years of observation, near Lawrence,
Kansas, the first storms to bring large numbers of males to the breeding
ponds occurred on June 20, 1947, June 18, 1948, and May 1, 1949.
In 1954 the frogs were recorded first on April 25, but these were
under massive boulders, and were still semi-torpid. Frogs were
found fully active, in numbers, under small flat rocks on May 7.
They were found frequently thereafter. On the afternoon of May
13, the third consecutive day with temperature slightly above 21° C.,
low croaking of a frog was heard among rocks at an old abandoned
quarry. Throughout the remainder of May, calling was heard frequently
at the quarry on warm, sunny afternoons. Often several
were calling within an area of a few square yards, answering each
other and maintaining a regular sequence. In the last week of May
rains were frequent, and the precipitation totalled 2.09 inches. On
June 1 and 2 also, there were heavy rains totalling 2.26 inches. On
the evening of June 2 many frogs were calling at a pond ½ mile south
of the Reservation, and one was heard at the pond on the Reservation.
By the evening of June 4, dozens were calling in shallow water
along the edge of this pond in dense Polygonum and other weeds.
There was sporadic calling even in daylight and there was a great[Pg 286]
chorus each evening for the next few days, but its volume rapidly
diminished.
In mid-June a system of drift fences and funnel traps was installed
200 yards west of the pond in the dry bottom of an old diversion
ditch leading from the pond. The ditch constituted the boundary
between bottomland pasture and a wooded slope, and therefore was
a natural travelway. The object of the installation was to intercept
and catch small animals travelling along the ditch bottom. The
drift fence was W-shaped, with a funnel trap at the apex of each
cone so that the animals travelling in either direction would be
caught. The numbers of frogs caught from time to time during the
summer provided information as to their responses to weather in
migrating to the pond.
Table 1. Numbers of Frogs Caught Within Two Days After Rain in Funnel
Traps in 1954, from Mid-June, to the Time of First Frost.
| Date | Precipitation in inches | No. of caught frogs |
|---|---|---|
| July 1 | 2.02 | 8 |
| July 10 | .11 | none |
| July 16 | 1.26 | none |
| July 20-21 | .94 | 3 |
| July 24 | .38 | 2 |
| July 28 | .29 | none |
| August 1-2 | 3.22 | 31 |
| August 6-7-8 | 2.43 | none |
| August 12 | .28 | none |
| August 16 | .29 | none |
| August 19-22 | .70 | none |
| August 27-28 | 1.05 | none |
| September 9 | .50 | none |
| September 29-30 | .38 | none |
| October 4 | .74 | none |
| October 12-14 | 3.51 | none |
From the positions of the traps and drift fences, it was obvious
that all of the frogs that were caught were travelling toward the
pond. Capture of an equal number moving away from the pond a
few days afterward might have been expected but none at all was
caught while making a return trip. Therefore it seems that the
frogs returned by a different route to their home ranges after breeding.
Of necessity they make the return trip under conditions drier
than those that prevail on the pondward trip, which is usually made
in a downpour. Probably the return travel is slower, more leisurely,
and with more tendency to keep to sheltered situations.
The call is a bleat, resembling that of a sheep, but higher, of lesser
volume, and is not unlike the loud rattling buzz of an angry bee.
The call is usually of three to four seconds duration, with an interval[Pg 287]
several times as long. Calling males were floating, almost upright,
in the water within a few yards of shore, where there was dense
vegetation. The throat pouch when fully expanded is several times
as large as the entire head. When a person approached to within
a few yards of frogs they usually stopped calling, submerged, and
swam to a place of concealment.
Having heard the call of typical G. carolinensis in Louisiana, I
have the impression that it is a little shorter, more sheeplike, and less
insectlike than that of G. olivacea. The call of Gastrophryne is of
such peculiar quality that it is difficult to describe. Different observers
have described it in different terms. Stebbins (1951: 391)
has described the call in greatest detail, and also has quoted from
the descriptions of it previously published. These descriptions include
the following: "high, shrill buzz"; "buzz, harsh and metallic";
"like an electric buzzer"; "like bees at close range but more like
sheep at a distance"; "bleating baa"; "shrill, long-drawn quaw quaw";
"whistled whēē followed by a bleat."
Stebbins observed breeding choruses (mazatlanensis) at Peña
Blanca Springs, Arizona, and stated that sometimes three or four
called more or less together, but that they seldom started simultaneously.
Occasionally many voices would be heard in unison followed
by an interval of silence, but this performance was erratic.
At the pond on the Reservation I noted this same tendency many
times. After a lull the chorus would begin with a few sporadic
croaks, then four or five or even more frogs would be calling simultaneously
from an area of a few square yards. Anderson (op. cit.:
34) found that in small groups of calling G. carolinensis there was
a distinct tendency to maintain a definite pattern in the sequence of
the calls. One "dominant" individual would initiate a series of calls,
and others each in turn would take up the chorus.
Pairing takes place soon after the breeding aggregations are
formed. On the night of June 4, 1954, a clasping pair was captured
and kept in the laboratory in a large jar of water. This pair did not
separate, and spawning occurred between noon and 1:30 P. M. on
June 5. When the newly laid eggs were discovered at 1:30 P. M.
most of them were in a surface film. Some were attached to submerged
leaves and a few rested on the bottom. The pair was still
joined, but the male was actually clasping only part of the time, and
as the frogs moved about in the water, it became evident that they
were adhering to each other by the areas of skin contact, which were
glued together by their dermal secretion. They were unable to
separate immediately, even when they struggled to do so. They[Pg 288]
were observed for approximately 15 minutes before separation occurred,
and during this time they were moving about actively. As
they separated, the area of adhesion was discernible on the back of
the female. It was U-shaped, following the ridges of the ilia and
the sacrum.
On August 2, 1954, after a rain of 3.22 inches, the previously mentioned
funnel trap in the ditch had caught 31 ant-eating frogs.
Water had collected to a depth of several inches in the depression
where the trap was situated. A dozen of the trapped frogs were
clasping pairs. These frogs struggled vigorously as they were removed
from the traps, handled and marked. As a result most of
the clasping males were separated from the females. In handling
those of each pair I noticed that they were glued together by dermal
secretions, as were those of the pair observed on June 5. The areas
of adhesion were of similar shape and location in the different pairs,
and included the U-shaped ridge of the female's back and the male's
belly, and the inner surfaces of the male's forelegs with the corresponding
surfaces of the female's sides where the male clasped.
This adhesion of the members of a pair during mating may be a
normal occurrence. The copious secretion of the dermal glands is
of especially glutinous quality in Gastrophryne. The adhesion of
members of a pair may have survival value. These small frogs are
especially shy, and in the breeding ponds they respond to any disturbance
with vigorous attempts to escape and hide. Under such
circumstances the adhesion may prevent separation. Also, it may
serve to prevent displacement of a clasping male by a rival. Anderson
(op. cit.) who observed many details of the mating behavior of
G. carolinensis, both in the laboratory and under natural conditions,
mentioned no such adhesion between members of a pair.
Anderson (op. cit.: 31) discussed the possibility that reproductive
isolation might arise in sympatric populations, such as those of G.
carolinensis in southern Louisiana, through inherent differences in
time of spawning. However, in G. olivacea at least, such isolation
would be prevented by individual males returning to breed at different
times in the same season. Furthermore, individual differences
in choice of breeding time probably result from environmental factors
rather than genetic factors in most instances. In G. olivacea
in Kansas, time of breeding is controlled by the distribution of heavy
rainfall creating favorable conditions. Onset of the breeding season
may be hastened or delayed, or an entire year may be missed because
of summer drought. If favorable heavy rains are well distributed
throughout the summer, frogs of age classes that are not yet[Pg 289]
sexually mature in the early part of the breeding season, may comprise
the bulk of the breeding population in late summer.
DEVELOPMENT OF EGGS AND LARVAE
Eggs laid on June 5 by the pair kept in the laboratory were hatching
on June 7, on the average approximately 48 hours from the time
of laying. By June 8 all the eggs had hatched and the tadpoles were
active. On August 28 and 29 thousands of newly metamorphosed
young were in evidence on wet soil at the pond margin; in some the
head still was tadpolelike and they had a vestige of the tail stump.
These young were remarkably uniform in size, 15 to 16 mm. (the
smallest one found was 14½ mm.) and almost all of them had originated
from eggs laid after heavy precipitation, totalling 3.22 inches,
in the first 36 hours of August. Allowing one day for adults to reach
the pond and spawn, and two days more for eggs to hatch, the tadpole
stage must have lasted approximately 24 days in this crop of
young.
Wright and Wright (1949: 582) stated that the tadpoles metamorphosed
after 30 to 50 days, and that the newly metamorphosed
frogs are 10 to 12 mm. in length. Length of time required for larval
development probably varies a great deal depending on the interaction
of several factors such as temperature and food supply.
GROWTH
Little has been recorded concerning the growth rate of Gastrophryne
or the time required for it to attain sexual maturity. Wright
(1932) found that G. carolinensis in the Okefinokee Swamp region
has a mean metamorphosing-size of 10.8 mm. Young thought to
be those recently emerged from their first hibernation were those in
the size group 15.0 to 20.0 mm., while the frogs in the 20 to 27 mm.
size class and those in the 27 to 36 mm. class were interpreted as
representing two successively older annual age classes. Anderson
(1954: 41) thought he could recognize four successive annual age
classes in the same species in southern Louisiana. He found that
sexual maturity is attained at a length of 21 to 24 mm. in frogs which
he believed to be late in the second year of life.
Allowing for size differences between the two species, Wright's
and Anderson's conclusions regarding growth in G. carolinensis, on
the basis of size groups, are largely substantiated by my own data
on the growth of marked individuals of G. olivacea living under
natural conditions in Kansas.
In 1954, an opportunity to investigate the early growth was afforded[Pg 290]
by unusually favorable circumstances. The population of
frogs that emerged from hibernation in the late spring of 1954 included
few, if any, that were below adult size; drought had prevented
successful breeding in 1952 and 1953. Heavy rains in the
first week of June, 1954, and again in the first week of August, resulted
in the production of two successive crops of young so widely
spaced that they were easily distinguishable. Some young may have
been hatched after other minor rains, but certainly these were relatively
few. Young from the eggs laid in the first week of August
were metamorphosing during the last week of August. Growth in
the frogs of this group can be shown by the average size and the size
range of the successive samples collected.
Table 2. Growth in Frogs Metamorphosed in the Last Week of
August, 1954.
| Time of sample | Number in sample | Mean size in mm. | Size range in mm. |
|---|---|---|---|
| August 27 to 31 | 27 | 15.55 ± .079 | 15 to 17 |
| September 11 | 114 | 17.2 ± .033 | 14 to 20 |
| September 15 to 22 | 12 | 18.7 ± .090 | 16 to 20 |
| September 27 to 30 | 37 | 19.3 ± .055 | 17 to 21.5 |
| October 1 to 7 | 62 | 20.8 ± .072 | 17 to 24 |
| October 12 to 17 | 49 | 22.3 ± .092 | 18 to 24 |
By mid-October, six weeks after metamorphosis, these frogs had
increased in over-all length by approximately 50 percent. Having
grown a little more than 1 mm. per week on the average, they were
approximately intermediate in size between small adults and newly
metamorphosed young.
The frogs hatched in June were present in relatively small numbers
compared with those hatched in August, and were not observed
metamorphosing. In late August a sample of 33 judged to belong
to the June brood averaged 26.2 (22-28) mm. long. A sample of
39 from the first week of October averaged 28.1 (24.5-32) mm.
Frogs of this group thus were approaching small adult size late in
their first growing season. Such individuals possibly breed in the
summer following their first hibernation, when they are a year old or
a little more. Because recaptured frogs were not sacrificed to determine
the state of their gonads, the minimum time required to attain
sexual maturity was not definitely determined. The available
evidence indicates that sexual maturity is most often attained late[Pg 291]
in the second year of life, at an age of approximately two years. The
darkened and distensible throat pouch of the adult male probably
is the best available indicator of sexual maturity.
Fig. 3. Growth shown by successive samples of young ant-eating frogs of two
size groups in late summer and early fall of 1954. For each sample the mean,
standard deviation, and range are shown. Lower series are those metamorphosed
in late August, and upper series are those metamorphosed in late
June.
[Pg 292]
Fig. 4. Rapid growth of a young female caught in June, July, and August,
1949. Presumably this individual metamorphosed late in the summer of 1948,
and at the age of approximately one year it was near small adult size.
Frogs that metamorphose in late summer have little time to grow
before hibernating, and still are small when they emerge in spring.
The smallest one found was 19 mm. long (May 19, 1951), and in
each year except 1954 many such young were found that were less
than 25 mm. in length in May or early June. None of the frogs
marked at or near metamorphosing size has been recaptured, but
the trend of early growth is well shown by Table 2 and Fig. 3. However,
many juveniles that were captured and marked within a few
weeks of metamorphosis were recaptured as adults. The selected
individuals in Table 3 are considered typical of growth from "half-grown"
to small adult size. Growth in many other individuals is
shown in Figs. 6 and 7.[Pg 293]
Table 3. Growth in Frogs Marked as Young and Recaptured as Small
Adults.
| Individual and sex | Dates of capture | Length in mm. | Probable time of metamorphosis |
|---|---|---|---|
| No. 1 | August 28, 1951 | 21.5 | Mid-July, 1951 |
| May 5, 1952 | 23 | ||
| July 3, 1952 | 32 | ||
| August 31, 1952 | 33 | ||
| No. 2 | June 8, 1950 | 25 | Late July, 1949 |
| May 24, 1951 | 31 | ||
| July 30, 1951 | 34 | ||
| June 24, 1952 | 35 | ||
| No. 3 | August 31, 1951 | 24 | Late June, 1951 |
| May 23, 1953 | 32 | ||
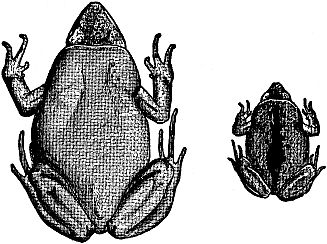
Fig. 5. Ant-eating frogs, a little less than twice natural size,
adult and newly metamorphosed young, showing differences
in size and coloration. The young is darker and has a leaflike
middorsal mark which fades as growth proceeds.
The trend of growth after attainment of minimum adult size is
also well shown by the records of marked individuals recaptured.
Many of these were marked while they were still small so that their
approximate ages are known. For those recaptured in their second
year, after one hibernation, length averaged 30.92 mm. Some of
this group were young metamorphosed late the preceding summer
and still far short of adult size (as small as 23 mm.) when recaptured.
Others were relatively large, up to 33 mm. A group of 22
recaptured frogs known to be in their third year averaged 33.3 mm.
(males 31.9, females 35.3, excluding four individuals of undetermined[Pg 294]
sex). Fifteen other recaptured frogs were known to be in
their fourth year at least, and some probably were older, as they
were already large adults when first examined. These 15 averaged
36.6 mm. (males 34.7, females 37.9 mm.). Size was similar in a
sample of 58 individuals intercepted en route to the breeding pond
in heavy rains of June and August, 1954. The 38 males in this
sample ranged in size from 30 mm. to 38 mm., averaging 34.5. The
20 females ranged from 34 mm. to 40 mm., averaging 37.65. The
large average and maximum size in this sample of a breeding population
may be typical after periods of drought years have prevented
successful reproduction. Summer drought in 1952 and 1953 prevented
breeding in those years, or, at least, it drastically reduced
the numbers of young produced. One-year-old and two-year-old
frogs may not have been represented at all in the sample of 58.
Three-year-old frogs presumably made up a substantial part of the
sample, since 1951 was a year of successful breeding.
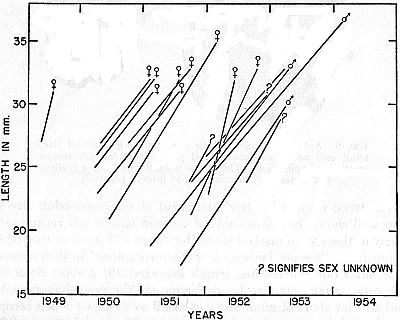
Fig. 6. Growth in a group of frogs, each marked while still short of adult
size and mostly recaptured after lapse of one or more hibernation periods.
Each line connects records of an individual frog.
Differences in size between species and geographic variation in
size in Gastrophryne have been given little attention by herpetologists,
but if understood, would help to clarify relationships. Hecht[Pg 295]
and Matalas stated in their revision (1946: 5) that size is of no importance
as a taxonomic character, as typical carolinensis, olivacea,
and mazatlanensis all averaged approximately the same—26 to 28
mm.—females slightly larger than males. However, they arbitrarily
classed as adults all individuals 22.5 mm. in length or larger, having
found individuals this small that showed the darkened and distensible
throat pouches characteristic of adult males. From the trend of
my own measurements of G. olivacea in northeastern Kansas, I conclude
that either many immature individuals were included in their
samples, or that the populations sampled included some with individuals
that were remarkably small as adults.
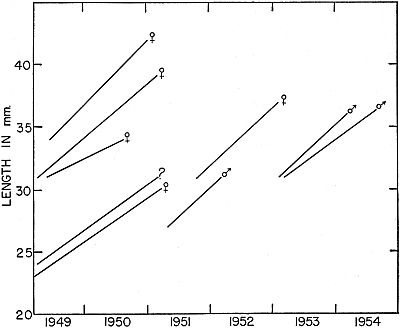
Fig. 7. Growth in another group of frogs that were marked as young or small
adults and recaptured after intervals of more than a year. Frogs of this group
were, on the average, larger than the individuals shown in Fig. 6, and they
made less rapid growth.
The population which I studied may be considered typical of
G. olivacea. They averaged large, including individuals up to 42
mm. in length, well above the maximum sizes for any reported in
the literature. At metamorphosis these olivacea are of approximately
50 percent greater length than G. carolinensis as reported
by Wright and Wright (1949: 573) and Anderson (1954: 41). Yet
Blair (1950: 152) observed that in eastern Oklahoma, where the[Pg 296]
ranges of olivacea and carolinensis overlap, the latter is larger. On
the basis of field and laboratory observations he tentatively concluded
that one of the main barriers to interbreeding was the reluctance
of the males of carolinensis to clasp the smaller females of
olivacea.
That size differs in different populations, and is still poorly understood,
is illustrated by the following discrepant figures from various
authors.
Table 4. Size Range of Adults in Various Populations of Gastrophryne.
| Species or subspecies | Geographic population sampled | Authority | Size range of adults in mm. |
|---|---|---|---|
| olivacea | Douglas Co., Kansas | present study | 31 to 42 |
| olivacea | entire range | Wright and Wright (1949) | 19 to 38 |
| carolinensis | entire range | Wright and Wright (1949) | 20 to 36 |
| carolinensis | southern Louisiana | Anderson (1954) | 22 to 35 |
| areolata | southeastern Texas | Wright and Wright (1949) | 23 to 29 |
| mazatlanensis | Arizona and New Mexico | Wright and Wright (1949) | 22 to 30 |
| mazatlanensis | Santa Cruz Co., Arizona | Stebbins (1951) | 25.2 to 31.5 |
COLOR AND PATTERN
The color pattern changes in the course of development, and the
shade of color changes in response to environmental conditions. At
the time of metamorphosis, young are dark brown with specks of
black and with a dark, cuneate, leaflike middorsal mark. The narrow
end of this mark arises just behind the head, and the mark extends
posteriorly as far as the hind leg insertions. At its widest,
the mark covers about half the width of the dorsal surface. The
lateral edges of the mark are sharply defined, but at its anterior and
posterior ends it blends into the ground color. In most individuals
smaller than 20 mm., this dorsal mark is well defined and conspicuous.
As growth proceeds, however, it becomes faint. In
frogs 19 to 25 mm. long the marks have disappeared. In individuals
of this size the brown ground color is markedly paler than in those
newly metamorphosed, but is darker than in adults.
In large adults the dorsal coloration is a uniform pale tan, paler
on the average in females than in males. Temperature and moisture[Pg 297]
both affect the shade of coloration. In frogs that were partly
desiccated, the color was unusually pale, with a distinctly greenish
tint, and at high temperatures coloration tended to be relatively pale.
Hecht and Matalas (1946) have described and figured color patterns
in various populations of Gastrophryne, demonstrating geographic
trends and helping to clarify relationships. Their account
indicates that the dark dorsal mark present in young of olivacea but
not present in adults, is better developed and longer persisting in
other forms. Specimens of carolinensis, presumably adult, are
figured which have the dark middorsal area contrasting with paler
color of the sides. The dark area is seen to consist of dots or blotches
of black pigment which may be in contact producing more or less
continuous black areas, or may be separate and distinct producing a
spotted pattern. Pigmentation is usually most intense along the
lateral edges of the dorsal leaflike mark; the central portion may be
so much paler that the effect is that of a pair of dorsolateral stripes.
This latter type of pattern is best developed in the population of
Key West, Florida. Hecht and Matalas did not consider these insular
frogs to be taxonomically distinct, because only 48 percent
of specimens from the Florida keys had the "Key West" pattern,
while 29 per cent resembled olivacea and 23 per cent resembled
carolinensis. In the southwestern subspecies (or species) mazatlanensis,
recorded from several localities in Sonora and from extreme
southern Arizona, the dorsal pigmentation similarly tends to
be concentrated in dorsolateral bands, but is much reduced or
almost absent, and there is corresponding pigmentation dorsally
across the middle of the thigh, across the middle of the shank, and
on the foot. When the leg is folded, these three dark areas are
brought in contact with each other and with the dorsolateral body
mark, if it is present, to form a continuous dark area, in a characteristic
"ruptive" pattern. Hecht and Matalas found similar leg bars,
less well developed, in certain specimens of olivacea including one
from Gage County, Nebraska, at the northern end of the known
geographic range.
MOVEMENTS
Freiburg (op. cit.: 384) concluded that ant-eating frogs seem
to have no individual home ranges, but wander in any direction
where suitable habitat is present. However, from records covering
a much longer span of time, it became increasingly evident that a
frog ordinarily tends to stay within a small area, familiar to it and
providing its habitat requirements.[Pg 298]
Nevertheless, in all but a few instances the marked frogs recaptured
were in new locations a greater or lesser distance from the
site of original capture. The movements made by these frogs were
of several distinct types:
- 1. Routine day to day movements from shelter to shelter within
the area familiar to the animal, the "home range." - 2. Shifts from one home range to another; such shifts may have
been either long or short, and may have occurred abruptly or
by gradual stages. - 3. Travel by adults to or from a breeding pond. In most or all
instances these adults were regularly established in permanent
home ranges, and they often moved through areas unsuitable
as habitat to reach the ponds. - 4. Movements of dispersal in the young, recently metamorphosed
and not yet settled in a regular home range.
Usually there was uncertainty as to which types of movements
had been made by the recaptured individuals. Some may have
made two or three different types of movements in the interval between
captures.
On many occasions individuals were found beneath the same rock
on two consecutive days, or occasionally on several successive days.
Rarely, such continued occupancy of a niche lasted several weeks.
In 1949, a frog was found under the same rock on June 4, 6, 26, 27,
and July 1, 3 and 11. This was an immature female, presumably
metamorphosed late in the summer of 1948. During the five weeks
period covered by the records, it grew from 27 mm. to 34 mm. In
1952, another individual was found under its home rock on June 23
and 30, July 2 and 3, and August 14 and 20. In 1952 a juvenile was
found under a rock on May 30, June 4, and June 17. These three
individuals were exceptional in their continued occupancy of the
same niches. Among the hundreds of others recorded, none was
found more than twice in any one place.
Despite the fact that field work was concentrated on small areas
which were worked intensively, only eight per cent of the frogs
recorded were ever recaptured, and most of those were recaptured
only once. Only 13 individuals yielded series of records, well
spaced, in two or more different years. These few individuals recaptured
frequently may not be typical of the entire population.
The low incidence of recaptures indicates that relatively few of the
frogs present on an area at any one time have been taken. Because
of their secretive and subterranean habits most of the frogs are
missed by a collector who searches by turning rocks, or trapping[Pg 299]
with pitfalls. Therefore, even though a marked frog may survive
and remain within a radius of a few hundred feet of one point for
months or even years, the chances of recapture are poor.
One female was caught first as a juvenile on June 8, 1950. On
April 24, 1951, when first recaptured, she had grown to small adult
size, and was only 18 feet from the original location. On July 30,
1951, however, she was recaptured 750 feet away. At a fourth capture
on May 21, 1952, she had shifted 70 feet farther in the same
direction. At the final capture on June 24, 1952, she was approximately
140 feet from both the third and fourth locations. The sequence
of these records suggests that the frog had already settled
in a home range at the time of her first capture in 1950, and that
approximately a year later she shifted to a second home range, which
was occupied for the following year, at least.

Fig. 8. Distances between captures in frogs marked, and recaptured after
substantial intervals including one or more hibernations. Distances are
grouped in 25-foot intervals. For longer distances the trend is toward progressively
fewer records, indicating that typical home ranges are small.
In several instances, after recaptures as far as 400 feet from the
original location, frogs were again captured near an original location,
suggesting that for some individuals, at least, home ranges may
be as much as 400 feet in diameter.
Figure 8 shows that for movements of up to 400 feet, numbers of
individuals gradually decrease with greater distance. For distances[Pg 300]
of more than 400 feet there are comparatively few records. Of the
59 individuals recaptured after one or more hibernations, only nine
had moved more than 400 feet from the original location. Twenty-five
were recaptured at distances of 75 feet or less. The mean distance
for movement for all individuals recaptured was 72 feet. A
typical home range, therefore, seems to average no more than 75
feet in radius. Of the 59 individuals recaptured after one or more
hibernations, 47 were adults and probably many of these had made
round-trip migrations to the breeding pond. This was not actually
demonstrated for any one individual, but several were captured
in each of three or four different years near the same location.
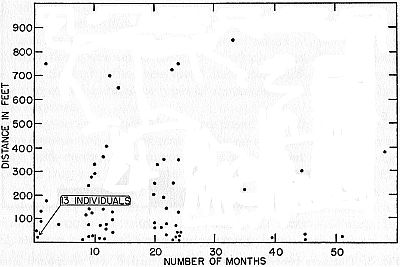
Fig. 9. Distances between captures and elapsed time in months in marked
frogs recaptured. Few records are for distances more than 400 feet. There
is but little tendency to longer movements in those caught after relatively
long intervals.
The trend of movements differed in the sexes. Males are more
vagile. Of 21 adult males recaptured, none was less than 40 feet
from its original location, whereas six of the 26 adult females were
less than 40 feet away from the original point of capture. Of seven
frogs that had wandered 700 feet or more, five were males.
FOOD HABITS
According to Smith (1934: 503) stomachs of many specimens,
from widely scattered localities in Kansas, contained only large numbers
of small ants. Tanner (1950: 47) described the situation of[Pg 301]
a frog found on the Reservation buried in loose soil beneath a flat
rock, beside an ant burrow, where, presumably, the frog could snap
up the passing ants without shifting its position. Anderson (op. cit.:
21) examined alimentary tracts of 203 specimens of carolinensis from
Louisiana, representing a year round sample for several different
habitats. He found a variety of small animals including ants, termites,
beetles, springtails, bugs, ear-wigs, lepidopterans, spiders,
mites, centipedes, and snails. Most of these prey animals were represented
by few individuals, and ants were much more numerous
than any of the other groups. Anderson concluded that ants, termites,
and small beetles were the principal foods. He noted that
some of the beetles were of groups commonly found in ant colonies.
Tanner reported that in a large number of the frogs which he collected
in Douglas, Riley, Pottawatomie, and Geary counties, Kansas,
the digestive tracts and feces contained only ants. Wood (1948:
226) reported an individual of G. carolinensis in Tennessee found
under a flat rock in the center of an ant nest.
Freiburg (op. cit.: 383) reported on the stomach contents of 52
ant-eating frogs collected near the Reservation. Ants constituted
nearly all these stomach contents, though remains of a few small
beetles were found. The ants eaten were of two kinds, Lasius interjectus
and Crematogaster sp. The latter was by far the more
numerous.
Although I made no further study of stomach contents, the myrmecophagous
habits of Gastrophryne have come to my attention frequently
in the course of routine field work. Individuals kept in
confinement for a day or more almost invariably voided feces which
consisted mainly or entirely of ant remains, chiefly the heads, as
these are most resistant to digestion.
Often upon examining frogs I have found ants (Crematogaster
sp.) or their severed heads, attached with mandibles embedded in
the skin. To have been attacked by ants, the frogs must have been
in or beside the ants' burrow systems. Frequently the frogs that
were uncovered beneath rocks were adjacent to clusters of ants or
to their nests or travelways, in a position strategically located to
feed upon them, as described by Tanner. Often the feces of the
frogs were found in pitfalls or under flat rocks. Although these
feces were not analyzed, they seemed to consist mainly or entirely
of ant remains.
The species of Crematogaster, which is the chief food of Gastrophryne
in this region, is largely subterranean in habits, and is extremely
abundant. Any flat rock in damp soil is likely to harbor[Pg 302]
a colony beneath it. Colonies are situated also in damp soil away
from rocks, beneath almost any kind of debris, and in hollow weed
stalks and decaying wood. Live-traps for small mammals, having
nest boxes attached, almost always were occupied by colonies of
Crematogaster, if they were left in the field in warm, humid weather.
Occasionally the ants attacked and killed small mammals caught in
such traps. Among the thousands of kinds of insects occurring on
the Reservation, this ant is one of the most numerous in individuals,
one of the most important on the basis of biomass and provides an
abundant food source for those predators that are ant eaters. Food
supply probably is not a limiting factor to populations of Gastrophryne
on the area.
PREDATION
Young copperheads are known to feed upon ant-eating frogs occasionally
(Anderson, 1942: 216; Freiburg, 1951: 378). Other kinds
of snakes supposedly eat them also. The common water snake
(Natrix sipedon) and garter snake (Thamnophis sirtalis) probably
take heavy toll of the adults at the time they are concentrated at the
breeding pools. Larger salientians may be among the more important
enemies of the breeding adults, the tadpoles, and the newly
metamorphosed young. Bullfrogs (Rana catesbeiana) and leopard
frogs (Rana pipiens) are normally abundant at the pond on the
Reservation. These large voracious frogs lining the banks are quick
to lunge at any moving object, and must take heavy toll of the much
smaller ant-eating frogs that have to pass through their ranks to
reach the water. The newly metamorphosed young often are forced
to remain at a pond's edge for many days, or even for weeks, by
drought and they must be subject to especially heavy predation by
ranid frogs. Even the smallest newly metamorphosed bullfrogs and
leopard frogs would be large enough to catch and eat them.
As a result of persistent drought conditions in 1952 and 1953, bullfrogs
were completely eliminated from the pond by early 1954.
Re-invasion by a few individuals occurred in the course of the
summer; these probably made long overland trips from ponds or
streams that had persisted through the drought. Leopard frogs
reached the pond in somewhat larger numbers, but their population
in 1954 was only a small percentage of that present in most other
years. Notable success in the ant-eating frog's reproduction in 1954
may have been due largely to the scarcity of these large ranids at
the breeding ponds.
Freiburg (loc. cit.) noted that many of the ant-eating frogs he[Pg 303]
examined were scarred, and some had digits or limbs amputated.
He did not speculate concerning the origin of these injuries. However,
it seems likely that many or all of them were inflicted by the
short-tailed shrew (Blarina brevicauda). Five-lined skinks living
on the same area were likewise found to be scarred by bites which I
identified (Fitch, 1954: 133) as bites of the short-tailed shrew.
This shrew is common on the Reservation, especially in woodland.
Many have been trapped in the pitfalls. On several occasions when
a short-tailed shrew was caught in the same pitfall with ant-eating
frogs, it was found to have killed and eaten them. Like the frogs,
the shrews were most often caught in pitfalls just after heavy rains.
Once in 1954 a shrew was found at the quarry in a pitfall that
had been one of those most productive of frogs. The bottom of the
pitfall was strewn with the discarded remains (mostly feet and
skins) of perhaps a dozen ant-eating frogs. All had been eaten during
one night and the following morning, as the trap had been
checked on the preceding day. On other occasions shrews caught
in pitfalls with several frogs had killed and eaten some and left others
unharmed.
SUMMARY
In northeastern Kansas the ant-eating frog, Gastrophryne olivacea,
is one of the more common species of amphibians. This area is
near the northern limits of the species, genus, and family. The species
prefers a dry, rocky upland habitat often in open woods or at
woodland edge where other kinds of salientians do not ordinarily
occur. It is, however, tolerant of a wide variety of habitat conditions,
and may occur in river flood plains or cultivated land. In
these situations where surface rocks are absent, cracks and rodent
burrows presumably furnish the subterranean shelter that it requires.
This frog is secretive and spends most of the time in subterranean
shelter, obtaining its food there rather than in the open. Only on
warm rainy nights is it inclined to venture into the open. Then, it
moves about rapidly and with a scuttling gait, a combination of
running and short hops. However, it may be flushed in daylight
from a hiding place by the vibrations from footsteps of a person or
an animal, or it may move about in the daytime when temperatures
at night are too low for activity. Though not swift of foot, the
frogs are elusive because of their tendency to keep under cover,
their slippery dermal secretion, and the ease with which they find
and enter holes, or crevices to escape.
Breeding occurs at any time from late May through August and[Pg 304]
is controlled by the distribution of rainfall. Heavy precipitation,
especially rains of two inches or more, stimulates the frogs to migrate
in large numbers to breeding ponds. Even though there are several
well spaced periods of unusually heavy rainfall in the course
of a summer, each one initiates a new cycle of migration, mating
and spawning. Heavy rainfall is a necessity, not only to ensure a
water supply in temporary pools where the frogs breed, but to
create the moist conditions they require for an overland migration.
An individual male may migrate to a pond and breed at least twice
in the same season. Whether or not the females do likewise is unknown.
Amplexus and spawning occur mainly within a day or two
after the frogs reach the ponds. The males call chiefly at night, but
there may be daytime choruses when breeding activity is at its
peak. Many males concentrate within a few square yards in the
choruses and float upright usually beside or beneath a stem or leaf,
or other shelter, rendering them extremely inconspicuous. The call
is a bleat of three seconds duration, or a little more. In amplexus
the members of a pair sometimes become glued together by their
viscous dermal secretions. The eggs hatch in approximately 48
hours. The tadpoles metamorphose in as few as 24 days. Newly
metamorphosed frogs are 15 to 16 mm. in length, or, rarely as small
as 14.5 mm. They are thus much larger than newly metamorphosed
G. carolinensis, which have been described as 10-12 mm. or even as
small as 8.5 mm. The newly metamorphosed frogs disperse from
the breeding ponds as soon as there is a heavy rain. The young
grow a little more than one mm. in length per week. Those metamorphosed
in early summer may attain minimum adult size before
hibernation which begins in October. It seems that sexual maturity
is most often attained in the second season, at an age of one to two
years.
Gastrophryne belongs to a family that is primarily tropical in distribution,
and frogs of this genus have much higher temperature
thresholds than most other amphibians of northeastern Kansas, with
a correspondingly short season of activity. For more than half the
year, mid-October to early May the frogs are normally in hibernation.
Body temperatures of active frogs ranged from 17.0° C. to
37.6° C., but more than two-thirds were within the relatively narrow
range, 24.0° to 31°. Near the date of the first autumn frost the frogs
disappear from the soil surface and from their usual shelters near
the surface, presumably having retired into hibernation in deep
holes and crevices.[Pg 305]
The natural enemies include young of the copperhead. The bullfrog
and leopard frog probably take heavy toll of both the adults
and the newly metamorphosed young at the breeding ponds. Reproductive
success of the ant-eating frogs was much greater in 1954
when these ranids were unusually scarce. The short-tailed shrew
is an important enemy. On occasion it took heavy toll of frogs
trapped in pitfalls, and many of the larger adults were scarred or
mutilated from bites, probably of the shrew.
Each of several frogs was found consistently under the same rock
for periods of weeks. The hundreds of other frogs that were marked
were rarely found twice in any one spot. Usually an individual recaptured
after weeks or months was still near the original site. In
many instances the distance involved was only a few yards, but
there is some evidence that home ranges may be as long as 400 feet
in greatest diameter. Of those caught in two or more different years
only 15 per cent were shown to have moved more than 400 feet.
These few exceptionally long movements, up to 2000 feet, involve
shifts in home range or migrations motivated by reproductive urge.[Pg 306]
LITERATURE CITED
Anderson, P.
1942. Amphibians and reptiles of Jackson County, Missouri. Bull. Chicago
Acad. Sci., 6: 203-220.
Anderson, P. K.
1954. Studies in the ecology of the narrow-mouthed toad, Microhyla
carolinensis carolinensis. Tulane Studies in Zool., 2: 15-46.
Blair, A. P.
1950. Note on Oklahoma microhylid frogs. Copeia, 1950: 152.
Bogert, C. M.
1949. Thermoregulation in reptiles, a factor in evolution. Evolution, 3:
195-211.
Bragg, A. N.
1943. Observations on the ecology and natural history of Anura, XV.
The hylids and microhylids in Oklahoma. Great Basin Nat., 4: 62-80.
de Carvalho, A. L.
1954. A preliminary synopsis of the genera of American microhylid frogs.
Occas. Papers Mus. Zool. Univ. Michigan, no. 555: 19 pp., 1 pl.
Dice, L. R.
1923. Notes on the communities of vertebrates of Riley County, Kansas,
with especial reference to the amphibians, reptiles and mammals.
Ecology, 4: 40-53.
Fitch, H. S.
1954. Life history and ecology of the five-lined skink, Eumeces fasciatus.
Univ. Kansas Publ. Mus. Nat. Hist., 8: 1-156.
Freiburg, R. E.
1951. An ecological study of the narrow-mouthed toad (Microhyla) in
northeastern Kansas. Trans. Kansas Acad. Sci., 54: 374-386.
Hecht, M. K., and Matalas, B. L.
1946. A review of the Middle American toads of the genus Microhyla.
American Mus. Novitates, no. 1315: 1-21.
Loomis, R. B.
1945. Microhyla olivacea (Hallowell) in Nebraska. Herpetologica, 2:
211-212.
Mittleman, M. B.
1950. Miscellaneous notes on some amphibians and reptiles from the
southeastern United States. Herpetologica, 6: 20-24.
Parker, H. W.
1934. A monograph of the frogs of the family Microhylidae. British Mus.
(Nat. Hist.) London, vii + 208 pp., figs. 1-67.
Pope, C. H.
1931. Notes on amphibians from Fukien, Hainan, and other parts of
China. Bull. American Mus. Nat. Hist., 61: 397-611.
Schmidt, K. P.
1953. A check list of North American amphibians and reptiles. Univ.
Chicago Press, viii + 280 pp.
[Pg 307]
Smith, H. M.
1934. The amphibians of Kansas. American Midland Nat., 15: 377-528,
pls. 12-20, maps 1-24.
1950. Handbook of amphibians and reptiles of Kansas. Univ. Kansas
Publ. Mus. Nat. Hist. Misc. Publ., 2: 1-336 pp., 233 figs.
Smith, H. M., and Taylor, E. H.
1950. Type localities of Mexican reptiles and amphibians. Univ. Kansas
Sci. Bull. 33: 313-380.
Stebbins, R. C.
1951. Amphibians of western North America. Univ. California Press, xviii
+ 539 pp.
Tanner, W. W.
1950. Notes on the habits of Microhyla carolinensis olivacea (Hallowell).
Herpetologica, 6: 47-48.
Wood, J. T.
1948. Microhyla c. carolinensis in an ant nest. Herpetologica, 4: 226.
Wright, A. H.
1932. Life-histories of the frogs of Okefinokee Swamp, Georgia. Macmillan
Co., New York, N. Y.
Wright, A. H., and Wright, A. A.
1949. Handbook of frogs and toads of the United States and Canada.
Comstock Publ. Co., Ithaca, New York.
Transmitted February 28, 1955.
Transcriber's Notes
A small number of inconsistencies and typographical errors have been
changed in the text:
- p. 279 "near-by" changed to "nearby" (in nearby counties of Kansas)
- p. 289 "successivly" changed to "successively" (two successively older annual age classes)
- p. 297 "per cent" changed to "percent" (only 48 percent of specimens from the Florida keys)
- p. 303 "famliy" changed to "family" (the northern limits of the species, genus, and family.)
Featured Books

Descriptions of Two Species of Frogs, Genus Ptychohyla
William Edward Duellman
erning their relationships.Taylor (1944) proposed the generic name Ptychohyla for a newspecies of fr...

Birds, Illustrated by Color Photography, Vol. 2, No. 5
Various
ct in my memory as if it hadoccurred this very day, I have thoughtthousands of times since, and will...

Zoological Illustrations, Volume 2
William Swainson
innamon Crabeater. Generic Character.—See Pl. 26.Specific Character.H. cæruleo viridis; pileo, co...

Herbert Carter's Legacy; Or, the Inventor's Son
Jr. Horatio Alger
W TEMPLE CHAPTER XXIII -- TEMPLE THE TEMPTER CHAPTER XXIV -- JAMES IS SNUBBED CHAPTER XXV -- THE ...

Martin Rattler
R. M. Ballantyne
ree other boys, the sons of a distant relative, who boarded with him; and he had lived forty years i...

Light O' the Morning: The Story of an Irish Girl
L. T. Meade
XVI. — TEN POUNDS. CHAPTER XXVII. — ADVENTURES—AND HOME AGAIN. CHAPTER XXVIII. — THE ...

The Swoop! or, How Clarence Saved England: A Tale of the Great Invasion
P. G. Wodehouse
THE GREAT BATTLE Chapter 10 THE TRIUMPH OF ENGLAND Chapter 11 ...

Spacehounds of IPC
E. E. Smith
WESSO CHAPTER I The IPV Arcturus Sets Out for MarsA narrow football of steel, the Interplanet...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "A Field Study of the Kansas Ant-Eating Frog, Gastrophryne olivacea" :