The Project Gutenberg eBook of Phylogeny of the Waxwings and Allied Birds
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Phylogeny of the Waxwings and Allied Birds
Author: M. Dale Arvey
Release date: December 3, 2010 [eBook #34556]
Most recently updated: January 7, 2021
Language: English
Credits: Produced by Chris Curnow, Tom Cosmas, Joseph Cooper, The
Internet Archive for some images and the Online Distributed
Proofreading Team at https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK PHYLOGENY OF THE WAXWINGS AND ALLIED BIRDS ***
The text herein presented was derived from scans of the original report
which were OCRed and proofread. Minor typographical errors (genus name
initial not italicized, missing parenthis, missing or superfluous
commas, etc.) were made but are not noted here. With the exception of
those corrections and those noted below, it is the same text.
One additional note, many of the figures list notation such as "× 1/2"
to denote that the image is shown at half the actual size. The images
reproduced herein most likely will not match the original printed scale due
to the display resolution used by the viewer.
| Page 480 : | Luis Potosí => Luís Potosi |
| Page 481 : | Luis Potosí => Luís Potosi |
| Page 481 : | Measureemnts => Measurements |
| Page 486 : | cedorum => cedrorum |
| Page 496 : | Luis => Luís |
| Page 516 : | Gatrocnemius => Gastrocnemius |
[Cover]
and Allied Birds
M. DALE ARVEY
Museum of Natural History
Volume 3, No. 3, pp. 473-530, 49 figures in text, 13 tables
October 10, 1951
University of Kansas
LAWRENCE
1951
[Pg 473]
and Allied Birds
M. DALE ARVEY
Museum of Natural History
Volume 3, No. 3, pp. 473-530, 49 figures in text, 13 tables
October 10, 1951
University of Kansas
LAWRENCE
1951
[Pg 474]
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, Edward H. Taylor,
A. Byron Leonard, Robert W. Wilson
Volume 3, No. 3, pp. 473-530, 49 figures in text, 13 tables
Published October 10, 1951
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1950
23-1019
[Pg 475]
Phylogeny of the Waxwings
and Allied Birds
by
M. DALE ARVEY
| PAGE | |
| Introduction | 476 |
| Acknowledgments | 476 |
| Nomenclatural History | 477 |
| Materials | 478 |
| Diagnoses | 478 |
| Coloration | 485 |
| Courtship | 489 |
| Nest Building | 491 |
| Food | 493 |
| Skeleton | 494 |
| Skull | 494 |
| Humerus | 499 |
| Pygostyle | 502 |
| Sternum | 505 |
| Relative Lengths of Bones | 505 |
| Leg-trunk Percentages | 509 |
| Arm-trunk Percentages | 511 |
| Musculature | 514 |
| Caudal Muscles | 514 |
| Pectoral Muscles | 517 |
| Hind Limb Musculature | 517 |
| Digestive Tract | 517 |
| Origin of the Species | 519 |
| Conclusions | 521 |
| Summary | 524 |
| Bibliography | 525 |
[Pg 476]
A small family of passerine birds, the Bombycillidae, has been
selected for analysis in the present paper. By comparative study of
coloration, nesting, food habits, skeleton and soft parts, an attempt
is made to determine which of the differences and similarities between
species are the result of habits within relatively recent geological
time, and which differences are the result of inheritance from
ancient ancestral stocks, which were in the distant past morphologically
different. On the basis of this information, an attempt is
made to ascertain the natural relationships of these birds. Previous
workers have assigned waxwings alone to the family Bombycillidae,
and a question to be determined in the present study is whether or
not additional kinds of birds should be included in the family.
It has generally been assumed that the nomadic waxwings originated
under boreal conditions, in their present breeding range, and
that they did not undergo much adaptive radiation but remained
genetically homogeneous. Also it is assumed that the species were
wide ranging and thus did not become isolated geographically to
the extent that, say, the Fringillidae did. The assumption that waxwings
originated in the northern part of North America or Eurasia
may be correct, but it is more probable that the origin was more
southerly, perhaps, in northern Mexico, of North America (see p.
519.) Subsequent to the differentiation of this stock in the south,
there was a northerly movement, while certain populations remained
behind and underwent an evolution different from the northern
group. Since the fossil record does not permit us to say when in
geological time the family originated, we must rely on anatomical
evidence and the distributional evidence of present-day species to
estimate when the family stock had diverged from some unknown
group sufficiently to merit the status of a separate family.
It is with pleasure that I acknowledge the guidance received in
this study from Professor E. Raymond Hall of the University of
Kansas. I am indebted also to Dr. Herbert Friedmann of the United
States National Museum for the loan of certain skins, skeletons,
and alcoholic material; to Mr. Alexander Skutch, for notes on certain
Central American birds; and to Dr. Henry W. Setzer, Mr.
George H. Lowery, Jr., Mr. Victor E. Jones, Mr. Victor Housholder,
[Pg 477]
Mr. Alvaro Wille-Trejos, and Mr. Morton F. Davis, for gifts of
specimens that have been used in this work. Suggestions and critical
comments from Professors Worthie H. Horr, Charles G. Sibley
and Edward H. Taylor are gratefully acknowledged. I wish also to
thank Mrs. Virginia Unruh for the preparation of the drawings used
in this work.
The oldest name available for any species of the waxwings is
Lanius garrulus Linnaeus (1758). Lanius garrulus and Lanius garrulus
variety B carolinensis were described as conspecific. The description
has been associated with the first of the two names. The
latter name is a nomen nudum since it was not accompanied by a
separate description. The generic name Lanius was originally applied
to both shrikes and waxwings by Linnaeus. Since that name
is applied to the shrikes only, the next available generic name that
may be applied to the generically different waxwings must be used.
This is Bombycilla, a name originally proposed by Brisson (1760)
for the Cedar Waxwing. In the 12th Edition of the Systemae Naturae
(1766) Gmelin proposed the generic name Ampelis for the
Bohemian Waxwing, and combined it with the specific name garrulus,
the Cedar Waxwing being termed variety B. Vieillot (1807)
proposed the generic name Bombycilla and combined it with a new
specific name, cedrorum, for the Cedar Waxwing. Vieillot has been
cited as the author of Bombycilla since that time, although Brisson
used Bombycilla 33 years before. Oberholser (1917) did not cite
Brisson's work in his discussion of the proper generic name for the
waxwings, and Bombycilla should be ascribed to Brisson and not
Vieillot, since Opinion 37, rendered by the International Zoölogical
Committee on Nomenclature, states that generic names used by
Brisson (1760) are valid under the Code. In consequence, the specific
name available for the Cedar Waxwing, since Brisson is ruled
not to be a binomialist, is Bombycilla cedrorum Vieillot (1807).
Most workers prior to 1900 utilized the family name Ampelidae to
include waxwings, silky flycatchers, and palm-chats. Ridgway
(1904:113) elevated the silky flycatchers to family rank under the
name Ptilogonatidae, and assigned the palm-chats to a separate
family, the Dulidae.
[Pg 478]
The following specimens, numbering 238, and representing each
currently recognized species and subspecies, were used in the study,
and were supplemented by observation in 1947 on specimens in the
United States National Museum.
| Species or Subspecies | Skin | Skeleton | Alcoholic |
| Phainoptila melanoxantha melanoxantha | 8 | 1 | 2 |
| Phainoptila melanoxantha minor | 2 | ||
| Ptilogonys cinereus cinereus | 13 | 3 | 4 |
| Ptilogonys cinereus molybdophanes | 6 | ||
| Ptilogonys caudatus | 16 | 3 | 4 |
| Phainopepla nitens nitens | 1 | 5 | |
| Phainopepla nitens lepida | 12 | 5 | 4 |
| Bombycilla cedrorum | 53 | 27 | 8 |
| Bombycilla garrula garrula | 4 | 3 | |
| Bombycilla garrula centralasiae | 9 | 2 | |
| Bombycilla garrula pallidiceps | 7 | 3 | 2 |
| Bombycilla japonica | 10 | | |
| Dulus dominicus dominicus | 9 | 5 | 2 |
| Dulus dominicus oviedo | 4 | 1 | |
| Totals | 153 | 54 | 31 |
Diagnosis.—Bill short, flat, somewhat obtuse, minutely notched near
tip of each maxilla, flared at base; gape wide and deeply cleft;
culmen convex; nasal fossa broad, exposed, or filled with short, erect
or antrorse, close-set velvety feathers; nostril narrowly elliptical;
rictal vibrissae long, short, or absent; lacrimal bone free,
articulating at two points; wings long and pointed, or short and
rounded; primaries ten, tenth reduced in some species; tail short,
narrow, even, two thirds or less length of wing, or much longer and
forked or rounded; feet weak (except in Dulus and Phainoptila);
tarsus generally shorter than middle toe and claw, distinctly
scutellate with five or six divisions, the lateral plate subdivided
(except in Phainoptila); lateral toes of nearly equal length; hallux
approximately as long as inner lateral toe, or shorter; basal phalanx
of middle toe more or less united to that of outer and inner toes;
body stout; head generally conspicuously crested; plumage soft, smooth
and silky (except in Dulus); eggs spotted; nest in trees; three
subfamilies, five genera, eight species.
Diagnosis.—Rictus with conspicuous bristles; nasal fossa almost
entirely exposed; tail long and rounded, graduated, or square; caudal
muscles and pygostyle well developed; wings rounded and short, first
primary a half to a third as long as second; second primary shorter
than third; humerus long,
[Pg 479]
with small external condyle; plumage soft and silky, less so in
Phainoptila; sexes dissimilar, young like adult female; three
genera, four species.
Phainoptila Salvin, Proc. Zoöl. Soc. London, 1877:367, April 17,
1877. Type Phainoptila melanoxantha Salvin.
Diagnosis.—Without crest; tarsus longer than middle toe and claw,
and booted or very slightly reticulate; tail shorter than wing,
rounded; nostril exposed, ovate; rictal bristles distinct; first
primary well developed; plumage normal, bill flared slightly at base.
Range.—Costa Rica and Panamá.
Phainoptila melanoxantha melanoxantha Salvin, Proc. Zoöl. Soc.
London, 1877:367; April 17, 1877.
Diagnosis.—Coloration of adult males: Pileum, hindneck, back,
scapulars, and upper tail coverts Black (capitalized color terms after
Ridgway, Color Standards and Color Nomenclature, Washington, D. C.,
1912), with Bluish Gray-Green gloss; rump Lemon Yellow tinged with
Olive; lower breast and abdomen Gull Gray or Slate Gray; sides and
flanks clear Lemon Yellow; lower chest, upper breast, and under tail
coverts Yellowish Olive-Green, extending to patch on sides and flanks
of same color; bill and feet Black or Blackish Brown. Coloration of
adult females: Most of upper parts Olive-Green, with Yellowish Olive
on rump; thighs Olive-Gray, as are sides of head; rest of coloration
as in male. Coloration of young: As in adult female, but duller
throughout.
Measurements.—Wing 99.0, tail 88.5, culmen 15.2, tarsus 28.4.
Range.—Highlands of Costa Rica and extreme western Panamá (Volcán
de Chiriquí).
Phainoptila melanoxantha minor Griscom, Amer. Mus. Novitates,
141:7, 1924.
Diagnosis.—Coloration as in P. m. melanoxantha, but female with
hindneck more extensively gray and of slightly darker shade; rump,
upper tail coverts, and edgings to tail feathers slightly greener,
less yellow; average size smaller than in P. m. melanoxantha.
Range.—Highlands of westeran Panamá (Cerro Flores and eastern Chiriquí).
Ptilogonys Swainson, Cat. Bullock's Mex. Mus., App. 4, 1824. Type
Ptilogonys cinereus Swainson.
Diagnosis.—Tail much longer than wing, even or graduated; head with
bushy crest; nostril large, rounded and fully exposed, bordered by
membrane; rictal bristles well developed; tarsus shorter than middle
toe with claw; plumage soft, blended.
Range.—Southwestern United States to Costa Rica.
[Pg 480]
Ptilogonys cinereus cinereus Swainson, Cat. Bullock's Mex. Mus.,
App. 4, 1824.
Diagnosis.—Coloration of adult male: Frontals, supralorals, malars,
and chin White; orbital ring White; auriculars and nape grayish brown;
rest of head smoke gray; back, scapulars, wing coverts, rump, and
upper tail coverts plain Bluish Black; rectrices (except middle pair)
with large patch of White midway between base and tip, rest plain
Bluish Black; chest, breast, and anterior parts of sides plain Bluish
Gray-Green, much lighter than back, and fading into paler Gray on
throat; abdomen and thighs White; flanks and posterior part of sides
Olive-Yellow or Yellowish Olive; under tail coverts Lemon Yellow;
bill, legs and feet Black. Coloration of adult females: Head plain
Smoke Gray, passing into White on frontals, malars, and chin; back,
scapulars, wing coverts, and rump Hair Brown; upper tail coverts Dark
Gull Gray; remiges and rectrices Black with faint Dusky Green gloss,
edged with Gull Gray; chest Dark Grayish Brown lightening to Wood
Brown on sides and flanks; abdomen White; under tail coverts Yellow
Ocher. Coloration of young: As in adult female, but paler throughout.
Measurements.—In adult male, wing 94.0, and tail 104.2; in adult
female, wing 93.3, and tail 94.8; both sexes, culmen 11.1, and tarsus
18.7.
Range.—Mountainous districts of central and southern Mexico, in
states of Durango, Zacatecas, Hidalgo, México, Oaxaca, Colima,
Morelos, Veracruz, San Luís Potosi, Guerrero and Michoacán.
Ptilogonys cinereus molybdophanes Ridgway, Man. N. American
Birds, 464 (footnote), 1887.
Diagnosis.—Coloration of adult male: Upper parts darker bluish than
in P. c. cinereus; venter paler; flanks Olive-Green rather than
Olive as in P. c. cinereus. Coloration of adult female: Like female
of P. c. cinereus but colors darker throughout; dorsum more
olivaceous.
Measurements.—In adult male, wing 89.4, and tail 97.1; in adult
female, wing 89.4, and tail 93.3; both sexes, culmen 11.7, and tarsus
17.3.
Range.—Western Guatemala, in subtropical and temperate zones.
Ptilogonys caudatus Cabanis, Jour. für Orn., 1866:402, Nov. 1866.
Diagnosis.—Coloration of adult male: Forehead and crown Pale
Grayish Blue, slightly paler anteriorly; orbital ring Lemon Yellow;
rest of head and neck, including crest, Olive-Yellow; throat paler and
tinged with Light Gull Gray; back, scapulars, rump, upper tail coverts
and wing coverts uniform Bluish Slate-Black; chest and breast similar
but paler; sides and flanks Yellowish Olive-Green; thighs, lower
abdomen, and under tail coverts Lemon Yellow; remiges, primary
coverts, and tail Black, glossed with Bluish Black and edged with Gull
Gray; inner webs of rectrices (except two middle pair)
[Pg 481]
with large middle patch of White; bill, legs, and feet Black.
Coloration of adult female: Forehead and crown Pale Gull Gray,
becoming paler anteriorly; rest of head, together with neck, back,
scapulars, rump, and wing coverts plain Yellowish Olive Green; chest
and breast similar but more grayish; lower abdomen and flanks White
tinged with Yellowish Olive; under tail coverts Olive-Gray; remiges,
primary coverts, and rectrices Black with Gull Gray edges. Coloration
of young: Dorsum plain Light Grayish Olive; upper tail coverts
Brownish Olive; underparts Grayish Olive anteriorly, becoming more
Yellowish Olive on abdomen; under tail coverts pale Yellowish Olive
with Grayish Olive base; bill and feet Brownish Drab.
Measurements—In adult male, wing 96.2, and tail 135.7; in adult
female, wing 93.9, and tail 113.7; both sexes, culmen 12.6, and tarsus
19.1.
Range.—Highlands of Costa Rica and extreme western Panamá.
Phainopepla Sclater, Proc. Zoöl. Soc. London, 26:543, 1858. Type
Phainopepla nitens (Swainson).
Diagnosis.—Tail almost as long as wing; head with pointed crest of
narrow, separated feathers; rectrices without white; bill narrow,
compressed terminally; conspicuous white patch under wing; nostril
small, exposed; rictal bristles distinct; tail slightly rounded.
Phainopepla nitens nitens (Swainson), Anim. in Menag., 1838:285,
Dec. 31, 1837.
Diagnosis.—Coloration of adult male: Uniform glossy Bluish Black;
inner webs of primaries except innermost pair with middle portion
White; bill, legs, and feet Black. Coloration of adult female: Plain
Olivaceous Black, longer feathers of crest Black, edged with Gull
Gray; remiges and rectrices Dusky Drab to Black; rectrices and coverts
margined by White; bill and feet Brownish Drab to Dusky Brown.
Coloration of young: Like adult female but more Brownish Drab.
Measurements.—No specimens examined; larger than P. n. lepida (Van
Tyne, 1925).
Range.—Central and southern Mexico, in states of Coahuila, San Luís Potosi,
Durango, Guanajuato, México, Puebla, and Veracruz.
Phainopepla nitens lepida Van Tyne, Occ. Pap. Bost. Soc. Nat.
Hist., 5:149, 1925.
Diagnosis.—Coloration same as P. n. nitens; separated by smaller
size.
Measurements.—Wing 91.0, tail 90.3, culmen 11.5, tarsus 17.6.
[Pg 482]
Range.—Southwestern United States, from central California,
southern Utah, and central western Texas southward to Cape San Lucas
in Baja California, and into northwestern Mexico (Sonora and
Chihuahua).
Diagnosis.—Wings long and pointed, reaching almost to tip of tail; first primary
spurious; second primary longest; tail short and even; rictal vibrissae
few and short; secondaries generally, and sometimes also rectrices, tipped with
red, corneous appendages; nasal fossa partly filled with short, antrorse, close-set
velvety feathers; plumage soft, silky; tail tipped with yellow band (red in B.
japonica); sexes alike; humerus short with large external condyle; caudal
muscles and pygostyle not well developed; bill flared widely at base; one genus,
three species.
Range of subfamily.—Holarctic breeding area; wanders nomadically south
in winter to Central America and West Indies, southern Europe and Asia.
Bombycilla Brisson, Orn. ii, 1760:337. Type Bombycilla garrula (Linnaeus).
Diagnosis.—As described for the subfamily.
Bombycilla cedrorum Vieillot, Hist. Nat. Amer., 1:88, Sept. 1, 1807
Diagnosis.—Coloration of adults: Shading from Saccardo's Umber on
dorsum to Bister on top of head; upper tail coverts and proximal rectrices
Gull Gray; underparts shade through pale Lemon Yellow wash on belly into
White on under tail coverts; forehead, lores, and eye-stripe Black; chin same,
soon shading into Blackish Mouse Gray and into color of breast; side of under
jaw with sharp White line; narrow line bordering forehead, and lores, White;
lower eyelid White; quills of remiges Dark Mouse Gray, darkening at tips;
inner quills tipped with red horny wax appendages; tail feathers like primaries,
but tipped with Lemon Yellow, and occasionally showing also red horny wax
appendages; bill and feet Black. Coloration of young: Dorsum as in adult,
but lightly streaked with White; head concolor with dorsum; forehead White;
lores Black; eye stripe Black anterior to eye and White posterior to eye;
throat Light Buff; belly with alternate streaks of Dresden Brown and light
Ochraceous Buff but posteriorly White; tail tipped with Lemon Yellow bar;
bill black at tip, shading to Sepia at base.
Measurements.—Wing 92.9, tail 55.5, culmen 10.9, tarsus 16.8.
Range.—Breeds from central British Columbia, central Alberta and Manitoba,
northern Ontario, southern Quebec and Cape Breton Island south to
northwestern California, northern New Mexico, Kansas, northern Arkansas,
North Carolina, and northern Georgia. Winters south to Louisiana, Mississippi,
Texas, Arizona, Colorado, Florida, Honduras, Costa Rica, Jamaica, Little
Cayman Island, Haiti, and Panamá.
Bombycilla garrula (Linnaeus), Syst. Nat., 10th Ed., 1758:55.
Diagnosis.—Coloration of adults: General color Olive-Brown, shading insensibly
from clear Smoke Gray of upper tail coverts and rump to Cinnamon-Drab
anteriorly, heightening on head and forehead to Hazel; narrow frontal
line, lores, broader mask through eye, chin, and upper throat, Sooty Black;
under tail-coverts Cinnamon-Brown; tail Smoke Gray, deepening to Blackish
[Pg 483]
Mouse Gray distally, and tipped with Lemon Yellow; wings Blackish Mouse
Gray; primaries tipped with sharp spaces of Lemon Yellow or White, or both;
secondaries with White spaces at ends of outer web, shafts usually ending with
enlarged, horny red appendages; primary coverts tipped with White; bill
Blackish Slate and paler at base; feet Black. Coloration of young: Much like
adult, but general color duller; some streaking on venter and back; chin,
throat, and malar region dull White. Three subspecies.
Bombycilla garrula garrula (Linnaeus), Syst. Nat., 10th Ed., 1758:55.
Diagnosis.—Coloration: As described for the species, but darkest of the
three subspecies; tending to be more Vinaceous dorsally than either pallidiceps
or centralasiae.
Measurements.—Wing 113.5, tail 63.1, culmen 12.5, tarsus 20.7.
Range.—Europe; breeds north to northern Russia and Norway, south to
about 65° N latitude; winters south to England and Ireland, southern France,
northern Italy, and Turkey.
Bombycilla garrula centralasiae Poljakov, Mess. Orn. vi:137, 1915.
Diagnosis.—Coloration: As described for the subspecies garrula, but less
Vinaceous dorsally, and more Cinnamon; venter lighter gray than garrula, and
much paler than pallidiceps.
Measurements.—Wing 114.7, tail 63.0, culmen 12.2, tarsus 21.0.
Range.—Asia; breeds northern Siberia south to Vladivostok; winters to
Turkestan and central eastern China and Japan.
Bombycilla garrula pallidiceps Reichenow, Orn. Monats. 16:191, 1908.
Diagnosis.—Coloration: As described for the species, but more grayish
above and below than B. g. garrula; darker gray than in centralasiae.
Measurements.—Wing 115.1, tail 71.7, culmen 12.6, tarsus 21.1.
Range.—Breeds from western Alaska to northern Mackenzie and northwestern
Manitoba south to southern British Columbia, southern Alberta,
northern Idaho, and possibly Colorado (Bergtold 1924) and Montana (Burleigh
1929); winters east to Nova Scotia and irregularly over much of Canada,
and south irregularly to Pennsylvania, Ohio, Michigan, Indiana, Kansas, Colorado,
California, Arizona, and Texas.
Bombycilla japonica (Siebold), Nat. Hist. Jap., St. No. 2:87, 1824.
Diagnosis.—Coloration: Dorsum generally Brownish Drab shading to Light
Brownish Drab on lower back, rump, and upper tail coverts; secondary and
tertiary coverts Pale Brownish Drab, washed on outer web with Carmine;
[Pg 484]
primary coverts Blackish Slate, with White edging; tail feathers Slate-Gray,
broadly tipped with Carmine, bordered anteriorly by subterminal Black bar;
head crested, forehead Chestnut; lores, frontals, and stripe extending around
eye and nape, Black; throat Black, narrowing on lower throat; breast, sides
of flanks Light Drab; venter pale Sulphur Yellow; thighs Brownish Drab;
under tail coverts Carmine; bill, legs, and feet Black.
Measurements.—Wing 108.3, tail 53.6, culmen 11.2, tarsus 19.4.
Range.—Breeds eastern Siberia, northern China; winters south in China,
and to Japan (Hokkaido, Kyushu), Taiwan, and Korea.
Diagnosis.—Bill deep and compressed, culmen strongly depressed; nostrils
circular, wholly exposed; tail even, and shorter than wing; tenth primary less
than half length of ninth; under parts streaked; plumage hard and harsh;
rictal bristles minute; wing rounded; humerus long and with small external
condyle; pygostyle and caudal muscles not well developed; one genus, one
species.
Range of subfamily.—Islands of Haiti and Gonave, Greater Antilles.
Dulus Vieillot, Analyse, 1816:42.
Diagnosis.—Like the subfamily.
Dulus dominicus dominicus (Linnaeus), Syst. Nat., 12th Ed., 1766:316.
Diagnosis.—Coloration: Dorsum Olive, back, scapulars, and wing coverts
more Brownish Olive; lower rump and upper tail coverts Olive-Green; pileum
and hindneck with indistinct streaks of Brownish Olive; tail Brownish Drab,
edged with Light Olive Gray; lores, suborbital region, and auricular regions
Dusky Brown; malars Dusky Brown and streaked with Sooty Black, streaks
narrower on abdomen, broader and paler on under tail coverts, bill Light
Brownish Drab; legs and feet Brownish Drab.
Measurements.—Wing 85.0, tail 68.8, culmen 15.0, tarsus 24.7.
Range.—Island of Haiti, Greater Antilles.
Dulus dominicus oviedo Wetmore, Proc. Biol. Soc. Wash., 42:117, 1929.
Diagnosis.—Coloration: Like D. d. dominicus, but averaging more Grayish
Olive; rump and tail coverts with less greenish wash.
Measurements.—Wing 90.1, tail 71.3, culmen 16.2, tarsus 25.1.
Range.—Gonave Island, off Haiti, Greater Antilles.
[Pg 485]
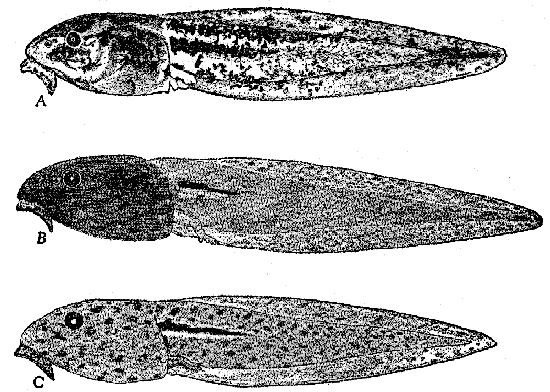
The general coloration of waxwings is cryptic, that is to say,
concealing or blending. The lighter color of the venter, especially of
the belly, contrasts with the duller, darker vinaceous color of the
dorsum. Several ruptive marks tend to obliterate the outline of the
body. The crest of the head, when elevated, tends to elongate the
body, making the outline less like that of a normal bird. The facial
mask effectively breaks up the outline of the head, and conceals the
bright eye, which would otherwise be strikingly distinct. The white
spots on the distal ends of the secondaries of B. garrula and the
yellow color on the distal ends of the rectrices (red in B.
japonica) are also ruptive. These ruptive marks on an otherwise
blending type of plumage might be important to waxwings, and probably
are more effective when the birds remain motionless in either a
well-lighted area or in one that is partly in shadow, rather than in
one that is wholly in shadow.
The red wax tips on the secondaries of the flight feathers, and
sometimes found on the ends of the rectrices in Bombycilla, are
puzzling and no wholly convincing reason has been suggested for their
occurrence. Two instances are known of yellow instead of red-colored
wax tips in B. cedrorum (Farley, 1924). It is well known that many
individuals, especially of B. cedrorum, do not possess these tips;
they are absent in a smaller proportion of individuals of B.
garrula. Of the 53 skins of B. cedrorum available in the University
of Kansas Museum of Natural History, which might be taken as a
sampling at random of the general population of this species, only 17
possess wax tips. A few specimens are unilateral, and the tips are of
varying sizes in different individuals. Of these 17 birds, 6 are
female and 7 male, the others being unsexed at the time of skinning.
This proportion is, roughly, half and half. Of the seven skins of B.
garrula pallidiceps in the same Museum, five possess the tips, and
two that are females have no trace of the red tips at all. Of the five
which do have the tips, two are males, two are females, and one is
unsexed. In a series of 13 specimens of the three subspecies of B.
garrula, loaned by the United States National Museum, all but two
individuals possess the tips on the secondaries, and, in addition,
four specimens, equally divided between the two sexes, have color on
the rachis of some rectrices, and small appendages of pigment extend
beyond the feathers. Stevenson (1882) found that among 144 specimens
of B. garrula garrula killed by storms in England in the winter of
1866-67, 69 individuals had
[Pg 486]
wax tips. Of these, 41 were males and 27 were females; the remaining
one was of uncertain sex. Among 38 definitely sexed B. garrula
pallidiceps in the California Museum of Vertebrate Zoölogy, Swarth
(1922:276) lists tips in 22 males and 16 females. These data indicate
that the proportion of birds with the wax tips is higher in B.
garrula than in B. cedrorum. The potentiality for wax tips is
possibly inherited according to Mendelian ratio.
Bombycilla japonica is of interest in that the adults, at least,
seldom have the waxy appendages. Nevertheless, in the specimens
observed, the entire distal ends of the feathers normally possessing
the tips in other species are suffused with red color. This may be the
original condition of all waxwings, or perhaps, instead, this species
is in a transitional stage in the development of the tips. Swarth
(1922:277) says concerning the probable derivation of the wax tips in
B. garrula (and in B. cedrorum): "the ornamentation, in fact, may
well have begun with the coloring of the shaft, spreading later over
adjoining feather barbs. The last stage would have been the coalescing
of the barbs, forming the waxlike scale as is now seen. Various steps
of this hypothetical development are supplied in the wing and tail
feathers of different birds of this series." Bombycilla japonica
thus may be close to the ancestral condition in the waxwing stock in
the development of the waxy appendage.
The rectrices of all three species of waxwings seldom possess the
wax tips, unless the secondaries have the maximum number of tips.
In these individuals, the pigment seems to "spill over" onto the tail
feathers. Eight is the maximum number of tips found on the secondaries.
Rectrices with wax tips are more frequently found in B. garrula,
and only occasionally in B. cedrorum. The pigment in the tip
of the tail of B. japonica is red rather than yellow as it is in the
other two species, and some individuals of the Japanese Waxwing
show a slight amount of coalescence of wax in the tail feathers as
well as in the secondaries.
If the tips were present in all members of the two species, it could
be postulated, in line with recent investigational work by Tinbergen
(1947), that the tips are in the nature of species "releasers,"
facilitating species recognition. Such recognition is now regarded as
of prime importance in the formation of species. It is improbable that
sex recognition may be aided, as there is no evidence to indicate that
the tips are found predominantly in either sex.
The wax tips are not limited to the adult birds in the species B.
garrula. Swarth (op. cit.) mentions the capture of several young
[Pg 487]
Bohemian Waxwings, and describes them as "possessing all the
distinctive markings of the most highly developed adult." This
includes wax appendages, and several citations are given (Wolley 1857,
Gould 1862) to indicate that this is the rule rather than the
exception, not only for the American subspecies pallidiceps, but at
least for the European subspecies garrula as well. On the other
hand, the young of B. cedrorum lack the wax tips, at least as far as
available data show.
Some characteristics of living animals are of the "relict" type; that
is to say, they were developed in ancient times when some unknown
ecological factor was operative which is no longer demonstrable, and
the characteristic is now neutral or at least not detrimental,
although of no positive value to the organism. Possibly the wax tips
of waxwings are thus to be explained. I am more inclined to the
opinion that the wax tips are adaptations to present-day ecological
conditions for the birds.
The wax tips are ruptive in effect, since the birds, especially in
winter, are habitués of bushes and trees that have berries, and the
tips, on the otherwise dull body, suggest berries. The red tips tend
further to disrupt the body outline at the midline, or slightly posterior
to this. Perhaps the wax tips on the rectrices emphasize the
end of the tail, the region of the body that is the least vital and that
may be expendable in times of pursuit by an enemy.
Any characteristic is of survival value to an organism if in any
way the characteristic enhances the chances of survival up to the
time when the organism can successfully raise even a few young to
maturity. If that character, as for example, the red wax tips on the
secondaries, helps to maintain the individual until it can raise to
independence a greater number than merely a few young, such a
character can be said to be of greater survival value. The character
may be effective for a brief period of time and may be uncommon; it
might be effective for a split second in time, and only at a particular
stage in the life history.
The winter period probably is the most hazardous for waxwings,
in that they then depend at times upon long flights to find food.
The food is vegetable, and thus is comparatively low in food value;
the birds must ingest large quantities of berries or dried fruits to
maintain themselves. In winter, in northern latitudes at least,
predators are more apt to prey upon those species which, like waxwings,
do not migrate south. The winter months are those in which
waxwings frequent berry bushes, and it may well be that in these
[Pg 488]
months, the wax tips that appear like berries, are especially valuable
to the birds, and operate selectively.
It is suggested, therefore, that the wax tips are of positive value to
waxwings, rather than being relict characters. Coalescence of pigment
has taken place in the formation of the wax tips. B. japonica is
closer to the ancestral stock insofar as wax tips are concerned, and
generally lacks the tips. B. cedrorum has the tips in approximately
half of the adults, and not at all in the young. B. garrula has the
tips in almost all the adults, and in a like proportion of the young,
and probably has evolved further in the development and retention of
the wax tips than has either of the other two species.
The streaked plumage of Dulus is decidedly generalized, and is
probably more nearly like the color of the ancestral stock. In this
connection it is notable that young Cedar Waxwings are streaked, and
young Bohemian Waxwings are streaked to a lesser degree. This
streaking is apparently a recapitulation of the feather color of the
stock. Perhaps the color of Dulus has not changed, as the streaking
would not be a disadvantage to the birds in their environment of light
and shadow. In joining together in groups and in the construction of
large communal nests, Dulus has evidently gained sufficient
protection against predators; other birds solve this problem by
modifying their coloration.
Ptilogonys is ruptively colored, but in a different fashion than
Bombycilla. The tail markings, the distinct yellow on the under tail
coverts, the sharply marked pileum, are all examples of ruptive
coloration. The generally lighter venter (especially under tail
coverts), the crest that may be elevated, and the generally drab
bluish dorsum, are cryptic and serve to hide the animal insofar as is
possible considering its habits. The very conspicuous coloration of
the male, in contrast to the more drab color of the female, however,
would lead one to believe that in Ptilogonys, following the pattern
of many passerine birds, the male leads a predator from the nest,
leaving the drab female to incubate the eggs, and thus preserve the
young.
It is difficult to suggest reasons for the brilliant coloration of the
male Phainopepla, unless it is for decoying predators away from
the nest. Possibly some birds survive not because of, but in spite
of, their coloration, and Phainopepla may be a case of this sort.
Anyone who has observed Phainopepla in life will agree, certainly,
that the male makes no attempt at concealment, and flaunts his
color to all comers.
[Pg 489]
The coloration of Phainoptila, in contrast to Phainopepla, is much
more plain, and is suited to its habits of brush dwelling; in a brush
habitat the drab coloration is difficult to detect. The Yellowish
Olive under tail-coverts and the Olivaceous dorsum are all evidences
of cryptic coloration, and undoubtedly, this bird depends upon hiding
for escape from its enemies, since it is a bird of the dense forest
cover.
Coloration, which varies relatively rapidly in response to differing
ecological conditions, has become more different in the species of
Bombycillidae than is true in many other families of passerine birds.
The explanation lies in early geographical isolation of the three
subfamilies, with consequent radiation in three directions. Waxwings
have become adapted by possessing a thick protective layer of feathers
and drab coloration broken by ruptive marks. They still retain the
streaked plumage, which is probably ancestral, in the juveniles; this
is lost at the first molt in the fall. In its evolution, Dulus has
developed large feet, heavy decurved beak, and the large communal nest
that affords protection from enemies; as a consequence, perhaps
Dulus did not need a plumage different from the primitive and
streaked one. The survival of Dulus may not have depended on either
ruptive marks or on brilliant and outstanding plumage. The large feet
and large bill seem to be responses to particular ecological
requirements, as will be shown later.
The Ptilogonatinae, with habits paralleling those of the flycatchers,
probably are considerably modified from the ancestral stock; the
coloration probably is more brilliant and conspicuous. Perhaps this
type of coloration and the habit of capturing insects from a perch are
correlated. Some amount of territoriality is characteristic of this
subfamily and dimorphism in color—the plumage of the male is
outstandingly conspicuous—possibly is of selective value to the race.
In a tropical forest community, a duller pattern possibly would be
more visible and thus would be selectively disadvantageous.
Waxwings are gregarious birds and individuals establish no
well-defined territories as do many birds. The nest itself is the only
defended territory, and as Crouch (1936) has shown, the Cedar Waxwing
will nest in close proximity to others of the same species. Swarth
(1932:275) mentions that the Bohemian Waxwing is tolerant of the nests
of other pairs near by. The extreme condition is that found in
Dulus, in which the territory is not limited even to
[Pg 490]
the nest, but to the individual compartment of the community nest.
Phainopepla, a less gregarious bird than Dulus and waxwings, has a
much more definite territory, although individuals of Phainopepla
are tolerant of others of the same species; no feeding territory is
established, and small flocks of birds feed together at any time of
the year.
In birds whose territories lack well-defined boundaries, it would be
expected that elaborate song would not have evolved, and that most of
the recognition of kind and sex would be dependent upon the behavior
of the birds. This is the fact; song, as such, is lacking in the three
subfamilies Bombycillinae, Ptilogonatinae, and Dulinae. Waxwings utter
(1) notes that serve to keep the flock together, (2) calls used by the
young in begging for food, and (3) some low notes that Crouch (op.
cit.:2) considered as possibly concerned with courtship.
Phainopepla has various call notes, and in addition, a succession of
notes which are run together. Ptilogonys utters a note which Skutch
(MS) characterizes as a loud, not unmusical "tu-whip" that is used as
the birds "fly in straggling parties which keep in contact by their
constant chatter." Dulus is described by Wetmore and Swales
(1931:349) as having only a variety of rather harsh chattering notes
in chorus.
The most notable behavior pattern associated with courtship in
Waxwings, in the absence of song, is the so-called "mating dance"
described by Crouch (1936), and observed by me in Lawrence, Kansas, in
the spring of 1948. This consists of one bird of a pair (presumably
the male) hopping along a branch toward the other bird (the female),
then away again, repeating the procedure for some little time. The
female remains motionless until, as the male approaches, mutual
fondling of the head and neck feathers takes place, or the birds may
peck at each other's bill. A berry may be passed from bill to bill,
although generally the berry is not utilized for food, and this can be
interpreted as a nervous reaction of the birds. It may be an instance
of "false feeding" as is seen in many birds, in which the female begs
for food, as a nestling would beg, as a preliminary to the sexual act.
I am of the opinion that these reactions are in the nature of
behavioristic patterns that bring the birds into the emotional balance
for copulation, as copulation follows the "dance." Sometimes, however,
copulation is preceded by a "nuptial flight" around the nesting area,
at which time the birds utter loud calls. Armstrong (1924:183) is of
the same opinion, citing numerous instances in which nuptial flights
and elaborate
[Pg 491]
displays have evolved for just this purpose. The birds are then in the proper
physiological balance to initiate the complicated sequence of
copulation, nesting, incubation, feeding, and brooding of the young.
It would be valuable to know more concerning the life histories of the
other birds considered in this paper, since behavior is inherent, and
probably can be cited as evidence of close relationship or the
opposite. All that I have been able to learn is that Phainopepla has
a nuptial flight in which the male chases the female, and that Dulus
(Wetmore and Swales, 1931:347) seeks the company of others of its kind
at all times, and that two birds, presumably paired, will sidle up to
one another when they are perched.
There are numerous papers concerning the nesting of waxwings. B.
garrula, owing to its nesting in the far north, where observers are
few, has received less attention than B. cedrorum. There is, on the
other hand, no literature that deals with the nesting habits of the
majority of the Ptilogonatines, with the exception of Phainopepla,
on which there is considerable literature (Merriam, 1896; Myers, 1907,
1908). No detailed study of the nesting of Dulus has been reported,
although Wetmore and Swales (1931) have described carefully the large
communal nest of this genus.
In Bombycilla, both members of a pair apparently aid in the
construction of the nest (Crouch, 1936; Swarth, 1932). Although the
sexes are alike in plumage and general appearance, most students of
the nesting of waxwings agree that one bird, assumed to be the female,
does most of the arranging of the material, and does the shaping of
the nest, whereas both birds carry materials to the nest site. As is
characteristic of many passerine birds, both members of the pair
gather materials and fly back to the nest site, where the female takes
the more active part in the construction of the nest itself.
Both species of American waxwings build bulky nests, with the
base or platform composed of a large amount of twigs and sticks,
from which there often trails a mass of sticks and moss or string.
Softer materials such as moss, plant fibers, and string, are placed
inside the platform; moss is readily available to, and preferred by,
B. garrula according to Swarth (op. cit.:271), and various plant
fibers and string are used by B. cedrorum. The inner lining consists
of soft plant fibers or down, dry grasses, and feathers. The nest is
usually unconcealed in a tree either adjacent to a trunk or on a main
[Pg 492]
side branch, but sometimes in a fork. Nest building by both Cedar
and Bohemian waxwings is rapid, taking from three to five days,
and is followed immediately by egg laying.
Nesting by waxwings is late in the season; June is the month
in which the nest is usually started. This is readily explainable in
Bohemian Waxwings, since adverse weather would prohibit earlier
nesting in the area in which they spend the summer. Crouch (op.
cit.:1) remarks that B. cedrorum possibly evolved in the far north
where it was impossible for it to start nesting earlier, and that the
habit has been retained. Perhaps, on the other hand, nesting is delayed
until the berry crop is ripe, to insure sufficient food for the
young.
Desertion of the nest is not uncommon in waxwings, despite the
tolerance to other animals that is shown by the birds. A new
nest may suddenly be begun before the first one is finished, and all
the materials from the first nest may be removed, or the nest may
be abandoned before it is completed. The eggs may be left at any
time up to hatching, and the young may be deserted, especially
in the earlier stages of development.
The very large and bulky communal nest of Dulus is not radically
different from the nest of waxwings. In the absence of sufficient
nesting sites, a pair of gregarious birds such as Dulus could combine
their nest with those of other pairs, retaining for their own
territory only the nest cavity, and in this way communal nests
might have evolved. The nest of Dulus is communal probably
because of the lack of suitable trees for nesting sites, and only incidentally
does this type of nest afford better protection from natural
marauders. Large numbers of Palm-chats work together in the
construction of the nest platform, and both sexes probably take
part in the work.
In Phainopepla the nest is built mostly by the male (Merriam,
1896; Myers, 1908), although the female does some of the work,
especially in the shaping and lining of the nest. In this genus, the
nest is usually a compact structure, but exceptional nests are of considerable
bulk. The nest is commonly placed in a fork near the main
trunk of a tree, in a conspicuous location, and generally is 10 to 20
feet from the ground. In shape and location, the nest closely corresponds
to that of Bombycilla, but the materials used for a base
are stems of annual plants, whereas Bombycilla uses more woody
twigs. The finer materials used by Phainopepla are more readily
obtainable in the ecological association inhabited by Phainopepla
than would be heavier twigs such as Bombycilla uses.
[Pg 493]
Waxwings are typically frugivorous; berries are the staple food.
The birds are known to catch insects, especially in the spring and
summer, and their insect gathering technique has been likened to
that of Tyrannid flycatchers. Nice (1941) experimented with a
young captive Cedar Waxwing and found that it had a decided
preference for red or blue berries, and that meal worms were utilized
as food only when the birds became educated by other captive birds
of other species as to the food value of the worms. Post (1916)
indicates that the food given to the nestlings of Cedar Waxwings is
entirely animal for the first three days, and that a mixed diet of
berries and insects is subsequently offered.
In feeding of the young, regurgitation of partly digested food does
not take place, according to Wheelock (1905). Rather, the adults
"store" food in the form of berries in the expanded esophagus or crop,
feeding them whole to the young. Digestion is an unusually rapid
process, involving merely minutes for the passage of berries and
cherries. This is correlated with a short intestinal tract, which is
unusual for a frugivorous bird. Nice's (1940) experiments with Cedar
Waxwings revealed that cherries would pass through the digestive tract
in 20 minutes, blueberries in 28 minutes, and chokecherries in 40
minutes. Heinroth (1924) states that berries pass through the
digestive tract of Bohemian Waxwings in the space of a "few minutes."
This rapid digestion is obviously adaptive, since the value of the
food is slight and therefore large quantities of it must be ingested;
the large seeds would hamper further ingestion until they were
eliminated, since they seem not to be regurgitated.
Members of the subfamily Ptilogonatinae are both insectivorous
and frugivorous insofar as available data show, although again there
is relatively little information available concerning them. Skutch
(MS) has found that the Guatemalan Ptilogonys cinereus catches
insects by repeated sallies into the air from a perch, after the manner
of flycatchers. He notes also that the birds feed on berries
of Eurya theoides and Monnina xalapensis. It is well known that
Phainopepla catches insects when these are available, and its liking
for berries is so apparent that in parts of its range, it is known as
the "pepper bird," since it frequents pepper trees (Schinus molle)
and feeds on the small red berries. The preserved specimens of
Ptilogonys and Phainoptila available for this study contain only
berries in the digestive tract. Dulus feeds mostly, if not wholly, on
plant food. According to Wetmore and Swales (1931:349), berries,
fruits, and parts of flowers are eaten.
[Pg 494]
A critical analysis of the skeletons provides evidence that aids
the student in estimating which differences are merely the result of
habits developed in relatively recent geological time as opposed to
those which owe their existence to more ancient heritage. Stresses
caused by the action of different sets of muscles can apparently
stimulate changes in bones to meet new needs, and the evidence from
genetics is that such mutations in wild birds are minute and cumulative,
rather than of large degree and of sudden appearance. Once
adaptive mutations have occurred, if genetic isolation from one
source or another accompanies it, a new population different from
the parental stock may become established. Study of the skeleton
of any species of living bird may indicate those characters identifiable
as modifications fitting it to a particular environment. If no
distinguishing characters are discovered that may be attributed to
environmental factors, such a species can be spoken of as generalized;
the inference then is that such a species is not modified for
a single, particular ecological niche.
Some parts of the skeleton, obviously, are more adaptable or
plastic than others. The beak seems to be the most adaptable part.
Probably this results from its frequent use; it is the part of the
bird to capture the food. The long bones, meeting the environment
as legs which serve as landing mechanisms or as locomotory appendages,
and as wings which provide considerable locomotion for
most birds, probably come next in order as regards plasticity. In
these parts, then, one may look for the most change in birds, which,
within relatively recent geologic times, have been modified to fit a
particular set of conditions. From the beak and long bones of a
species in which habits are unknown, one can infer the habits and
habitat from a comparison with the skeletal features of species of
known habits.
[↑ TOC]
Skull.—The skulls in all three subfamilies have essentially the
same general appearance and structure, the most marked differences
being, as would be expected, in the bills and associated bones.
The most specialized bill is to be found in Dulus; its bill is decurved,
and the associated bones are correspondingly changed for
support of the bill. For example, the palatines and "vomer" are much
wider, the palatines are more concave from below and have longer
posterior processes than the corresponding bones in Bombycilla.
Moreover, the "vomer" in Dulus and in Phainoptila is larger and
heavier than in Bombycilla, and the quadrate and pterygoid bones
are relatively large for support of the beak. The palatines, however,
[Pg 495]
are weak in Phainoptila. In the Ptilogonatinae, with the exception
of Phainoptila, the wings of the palatines flare more than in Bombycilla,
but not to the extent that they do in Dulus, nor does the palatine
bone present a concave appearance in the Ptilogonatinae. The
premaxilla is a relatively weak bone in Bombycilla and Phainopepla,
stronger in Ptilogonys, and is notably heavy in Phainoptila and
Dulus, and in these latter two genera shows a sharply-ridged tomium.
The maxillae connect to somewhat widened nasal and naso-lateral
processes in all the genera, and the premaxillae narrow
abruptly from this point forward. In the family, Phainopepla and
Phainoptila show the least flaring in this region.
 | |
 |  |
 |  |
 | 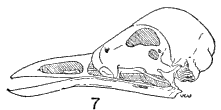 |
Figs. 1-7. Skulls in lateral view of five genera of Bombycillidae. Natural size.
| 1. | Phainoptila m. melanoxantha, sex?, MNH no. 26493, 15 mi. SE Cartago, Costa Rica. |
| 2. | Ptilogonys caudatus, male, MNH no. 24492, 15 mi. SE Cartago, Costa Rica. |
| 3. | Phainopepla nitens, male, MNH no. 24752, Pima Co., Arizona. |
| 4. | Ptilogonys cinereus, female, Louisiana State University no. 297, Xilitla Region, San Luís Potosi, Mexico. |
| 5. | Dulus dominicus, female, USNM no. 292652, Don Don, Haiti. |
| 6. | Bombycilla cedrorum, male, MNH no. 15331, Bexar Co., Texas. |
| 7. | Bombycilla garrula, sex?, USNM no. 223895, Bozeman, Montana. |
[Pg 496]
 | ||
 |  |  |
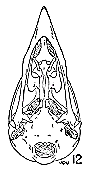 | 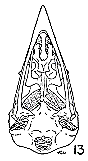 | 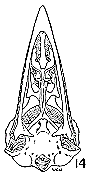 |
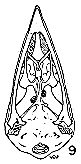
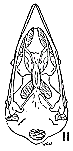
Figs. 8-14. Skulls in ventral view of five genera of Bombycillidae. Natural size.
| 8. | Phainoptila m. melanoxantha, sex?, MNH no. 26492, 15 mi. SE Cartago, Costa Rica. |
| 9. | Ptilogonys caudatus, male, MNH no. 24492, 15 mi. SE Cartago, Costa Rica. |
| 10. | Phainopepla nitens, male, MNH no. 24754, Pima Co., Arizona. |
| 11. | Ptilogonys cinereus, female, Louisiana State University no 297, Xilitla Region, San Luís Potosi, Mexico. |
| 12. | Dulus dominicus, female, USNM no. 292652, Don Don, Haiti. |
| 13. | Bombycilla cedrorum, male, MNH no. 15331, Bexar Co., Texas. |
| 14. | Bombycilla garrula, sex?, USNM no. 223895, Bozeman, Montana. |
[Pg 497]
 | ||
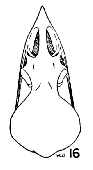 | 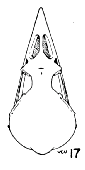 |  |
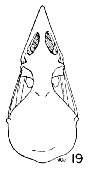 | 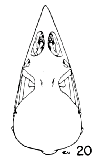 | 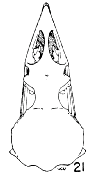 |
Figs. 15-21. Skulls in dorsal view of five genera of Bombycillidae. Natural size.
| 15. | Phainoptila m. melanoxantha, sex?, MNH no. 26493, 15 mi. SE Cartago, Costa Rica. |
| 16. | Ptilogonys caudatus, male, MNH no. 24492, 15 mi. SE Cartago, Costa Rica. |
| 17. | Phainopepla nitens, male, MNH no. 24752, Pima Co., Arizona. |
| 18. | Ptilogonys cinereus, female, Louisiana State University no. 297, Xilitla Region, San Luís Potosi, Mexico. |
| 19. | Dulus dominions, female, USNM no. 292642, Don Don, Haiti. |
| 20. | Bombycilla cedrorum, male, MNH no. 15331, Bexar Co., Texas. |
| 21. | Bombycilla garrula, sex?, USNM no. 223895, Bozeman, Montana. |
[Pg 498]
This flaring, immediately lateral to the antorbital plate, is common
to all Bombycillids and constitutes a major skeletal characteristic
useful for recognition of the members of the family, since the
swelling is easily discernible both externally and on the cleaned
skulls. In Phainopepla there is much variability in this character;
some specimens have a narrower antorbital bridge than others. Only
one skeleton of Phainopepla n. nitens was available. The flaring in
the skull of this specimen is identical with that in Ptilogonys.
Among the skulls of P. n. lepida in the University of Kansas Museum
of Natural History, is No. 19228, a juvenile, taken 5 miles
south of Tucson, Arizona. In this specimen, the flaring in the
antorbital region is clearly evident and equal in amount to that in
skulls of P. n. nitens, but the bird had not attained full skeletal
growth. However, the flaring of the antorbital region appears to
be common in the nestlings of many species of passerine birds.
Other specimens of the subspecies lepida show a varying amount of
flaring, the least (in the series available) being in No. 24754, MNH,
in which the proportion of the skull (length divided by width)
closely corresponds to that in Phainoptila; the skull of No. 24754 is
long and thin, and the base of the bill is only slightly swollen. The
skull of Phainopepla nitens lepida is more generalized than that of
Phainopepla n. nitens, having a longer and narrower bill like the
generalized Phainoptila. In Phainopepla n. nitens and in members
of the genus Ptilogonys, more flaring occurs in the antorbital region.
Phainoptila, as noted above, has no great amount of flaring in
the antorbital region. When more specimens of Phainoptila are
examined, the base of the bill probably will be found to flare more in
some individuals than in others; this would be expected if we may
judge by the data on Phainopepla. The premaxilla and maxilla of
Phainoptila are similar to the same bones in Dulus, and there is a
well-marked ridge on the tomium (possibly for cutting flower
parts). In Phainoptila, the palatines are narrower than in any other
genus of the family and abut the lacrimals. The entire skull appears
to be modified along different lines from those of the skull of
Dulus; the skull of Phainoptila seems to be modified for a frugivorous
rather than an insectivorous diet. The skull of Phainoptila
probably is more nearly similar to the ancestral skull than is that
of any other living species in the family. The wide gape characteristic
of some members of the family is undoubtedly a modification
for aiding in the capture of insects, and Phainoptila has progressed
less in this direction than have other species in the family.
[Pg 499]
The mandibles vary somewhat in the shape and proportionate
size of the bones. The mandible is proportionately, as well as actually,
highest in Dulus. The medial condyle varies to some extent,
being slightly flattened mediad in Bombycilla, and less so in the
other genera. The mandible of Bombycilla narrows to the symphysis
much more gradually than it does in the other genera.
The antorbital plate is large and divides the orbital chamber
from the nasal chamber. The small lacrimal bone anterior to the
plate articulates with the maxilla and the premaxilla. Shufeldt
(1889) states that the free lacrimal ossicle might be of some taxonomic
importance in the passerines, since it is found in the generalized
Corvids and in nestling Turdids. I find it well developed and
identical, with a double articulation and free ends, in all the Bombycillids.
There is no significant variability in the family, and this
is more evidence of close taxonomic relationship between the members
of the family.
The size of the crania is somewhat variable, although the differences
seem to be primarily those of proportion. Ptilogonatinae have
long crania, whereas the crania of the Bombycillinae and Dulinae
are shorter but deeper. I regard the longer cranium as primitive,
and it is longest in Phainoptila. In order of decreasing relative
length of the cranium, Phainoptila is followed by Ptilogonys caudatus,
P. cinereus, and Phainopepla. Bombycilla garrula has the
deepest cranium in the family.
The measurements of the lengths and widths of the skulls are given
in Table 9. The relative length of the bill and relative width of the
skull are given in Table 10. These relative measurements are calculated
by using the actual measurements in Table 9 as numerators,
the length of the skull from the lacrimal bone to the posteriormost
end of the skull being used as the denominator. The data indicate
that Phainoptila has a slightly narrower cranium.
[↑ TOC]
Humerus.—Certain families of passerine birds have a noticeable
variation in the characteristics of the humerus; the bone varies in
length, in diameter, and in the complexity of the processes at either
end. In the Bombycillids, however, the amount of variation is
relatively small, and the diaphysis of the bone is somewhat twisted,
especially so in Dulus. The deltoid tuberosity is variable, being
shorter but more elevated in Bombycilla than it is in the Ptilogonatinae
and in the Dulinae. The tendon from the pectoralis major
muscle, which inserts on this process, probably finds better insertion
on a higher process than on a lower but longer one.
[Pg 500]
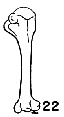 | ||
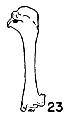 | 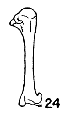 | 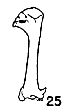 |
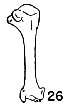 | 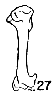 | 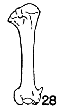 |
Figs. 22-28. Humeri of five genera of Bombycillidae. Natural size.
| 22. | Phainoptila m. melanoxantha, sex?, MNH no. 26493, 15 mi. SE Cartago, Costa Rica. |
| 23. | Ptilogonys caudatus, male, MNH no. 24492, 15 mi. SE Cartago, Costa Rica. |
| 24. | Phainopepla nitens, male, MNH no. 24754, Pima Co., Arizona. |
| 25. | Ptilogonys cinereus, female, Louisiana State University no. 297, Xilitla Region, San Luís Potosi, Mexico. |
| 26. | Dulus dominicus, female, USNM no. 292652, Don Don, Haiti. |
| 27. | Bombycilla cedrorum, male, MNH no. 15331, Bexar Co., Texas. |
| 28. | Bombycilla garrula, sex?, USNM no. 223895, Bozeman, Montana. |
Distally, the two major condyles and the intercondylar groove or
olecranon fossa that make efficient articulation with the ulnar process,
are not variable. The external condyle, however, is significantly
variable in the family. This condyle is longest and most pronounced
in birds in which the humerus is short in relation to the trunk, as for
example in Tachycineta. In the Bombycillidae the condyle is
smallest in Phainoptila, where it is a mere suggestion of a process.
In the remainder of the Ptilogonatinae, the condyle is larger but
rounded, and shows a double process in Ptilogonys caudatus, and a
slightly pointed process in P. cinereus. The external condyle in
Dulus is not specialized, being low and rounded, but in Bombycilla,
it is noticeably elongated, indicating a better attachment distally for
[Pg 501]
the deltoid muscle. (No measurements are tabulated for this
condyle, as the percentage of error in measuring this small structure
is great.) Table 1 gives lengths of humeri, and Table 2 gives
lengths of the humeri expressed as percentages of the length of the
trunk, a standard measurement.
The area of insertion of the deltoid muscle is elongated in those
birds with shortened humeri; these birds have also greater flight
power than do birds with longer humeri and therefore a shorter
external condyle.
Table 1. Lengths of Arm Bones in cm.
| Species | Humerus | Radius | Ulna | Manus |
| Ptilogonys caudatus | 2.39 | 2.57 | 2.79 | 2.25 |
| Ptilogonys cinereus | 2.24 | 2.48 | 2.78 | 2.38 |
| Phainopepla nitens | 2.21 | 2.59 | 2.82 | 2.39 |
| Phainoptila melanoxantha | 2.40 | 2.51 | 2.70 | 2.25 |
| Dulus dominicus | 2.23 | 2.38 | 2.63 | 2.31 |
| Bombycilla garrula | 2.35 | 2.58 | 2.88 | 2.67 |
| Bombycilla cedrorum | 2.06 | 2.34 | 2.60 | 2.38 |
Table 2. Arm-trunk Ratios (in percent)
| Species | Humerus | Radius | Ulna | Manus | Total |
| Ptilogonys caudatus | 85 | 92 | 93 | 80 | 2.58 |
| Ptilogonys cinereus | 84 | 90 | 103 | 89 | 2.76 |
| Phainopepla nitens | 84 | 98 | 107 | 91 | 2.82 |
| Phainoptila melanoxantha | 73 | 77 | 82 | 69 | 2.31 |
| Dulus dominicus | 78 | 83 | 92 | 81 | 2.51 |
| Bombycilla garrula | 69 | 75 | 87 | 78 | 2.34 |
| Bombycilla cedrorum | 67 | 76 | 85 | 77 | 2.29 |
[Pg 502]
Table 3. Arm-trunk Ratios (in percent)
| Species | Humerus | Radius | Ulna | Manus | Total |
| Corvus brachyrynchos | 90 | 101 | 111 | 106 | 307 |
| Dendroica audubonii | 68 | 82 | 90 | 77 | 237 |
| Setophaga ruticilla | 69 | 82 | 91 | 75 | 235 |
| Myadestes townsendi | 71 | 84 | 96 | 81 | 248 |
| Sialia sialis | 72 | 84 | 98 | 86 | 256 |
| Hylocichla mustelina | 75 | 81 | 92 | 80 | 247 |
| Parus atricapillus | 85 | 90 | 106 | 81 | 272 |
| Tachycineta thalassina | 71 | 95 | 107 | 128 | 306 |
| Myiarchus crinitus | 83 | 105 | 115 | 92 | 290 |
| Dumetella carolinensis | 76 | 75 | 89 | 78 | 243 |
| Polioptila caerulea | 85 | 93 | 105 | 71 | 261 |
| Eremophila alpestris | 91 | 99 | 110 | 95 | 296 |
| Muscivora forficata | 85 | 111 | 120 | 108 | 313 |
[↑ TOC]
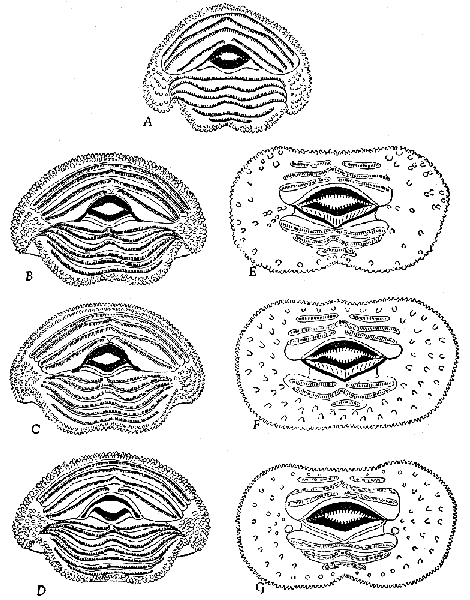
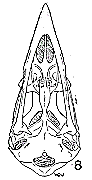
Pygostyle.—This part of the skeletal system is variable in the
species dealt with, not so much in size as in complexity. It reflects,
of course, the character of the caudal muscles and their size, as well
as the length of the rectrices and the corresponding force necessary
to hold these feathers upright and in a useful position. Firm attachment
is important even in flight, because the tail is used as a rudder,
and in the Ptilogonatinae as a brake. The pygostyle is most modified
in this subfamily.
In lateral aspect, the pygostyles of the species of the Ptilogonatinae
are similar. The crest of the bone is flattened dorsally, and
has a broad anterior surface that is thin and bladelike. This is
widest in Ptilogonys caudatus, and narrowest in Phainoptila, in
which genus, however, the entire bone is of small size. The centrum
is widest in Ptilogonys caudatus, and is progressively narrower in
P. cinereus, Phainopepla, and Phainoptila. Greater width provides
a larger area of attachment for the larger rectrices and also more
area for insertion of the lateralis caudae muscle, the size of which
varies more than that of the other caudal muscles in the different
species of the Bombycillidae.
[Pg 503]
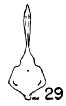 | |
 |  |
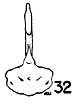 |  |
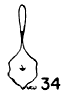 | 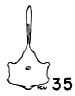 |
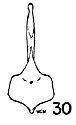
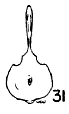
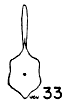
Figs. 29-35. Pygostyles in posterior view of five genera of Bombycillidae.
× 2.
| 29. | Phainoptila m. melanoxantha, sex?, MNH no. 26493, 15 mi. SE Cartago, Costa Rica. |
| 30. | Ptilogonys caudatus, male, MNH no. 24492, 15 mi. SE Cartago, Costa Rica. |
| 31. | Phainopepla nitens, male, MNH no. 24754, Pima Co., Arizona. |
| 32. | Ptilogonys cinereus, female, Louisiana State University no. 297, Xilitla Region, San Luís Potosi, Mexico. |
| 33. | Dulus dominicus, female, USNM no. 292652, Don Don, Haiti. |
| 34. | Bombycilla cedrorum, male, MNH no. 15331, Bexar Co., Texas. |
| 35. | Bombycilla garrula, sex?, USNM no. 223895, Bozeman, Montana. |
In proportionate size (see Table 7), the pygostyle of Bombycilla
is the smallest in the family. The dorsal spinous portion is acutely
pointed instead of flattened as in the Ptilogonatinae. In Dulus, the
spinous portion is extremely thin, and shows a decided curve dorsad
from the centrum, and there is no flattened area anterior to the
spinous portion as is seen in Ptilogonys.
The centrum in cross section varies considerably. In Bombycilla
the walls are indented, with definite terminal knobs; both knobs and
indentations are more pronounced in B. garrula than in cedrorum,
however. The spinous portion is enlarged in both species, and the
rest of the neck region is constricted (Figs. 29-35).
The centrum of Dulus in posterior aspect presents the appearance
of a simple shield; little of the indentation seen in Bombycilla is
[Pg 504]
present. The spinous portion is plain, with no constriction nor
terminal enlargement in the neck. The centrum in Phainopepla is
similar to that in Dulus, but has a small expansion at the base of the
spine, the entire centrum being wider in proportion to its over-all
size than in any of the other species mentioned previously. The
centrum in Ptilogonys shows great width, and the spine is in a
large expanded tip as in Bombycilla. The lateral edges of the centrum
in P. cinereus are "winged" and in two separate halves; whereas
the centrum of P. caudatus is fairly plain, its specialization being
reflected primarily in breadth and flatness. In cross section of the
centrum, Phainoptila is similar to Phainopepla, although, in the
former, the bone is smaller in proportion to the size of the animal,
and the lateral wings are more angular than in Phainopepla.
 | |
 |  |
 |  |
 | 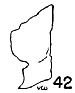 |
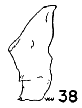
Figs. 36-42. Pygostyles in lateral view of five genera of Bombycillidae. × 2.
| 36. | Phainoptila m. melanoxantha, sex?, MNH no. 26493, 15 mi. SE Cartago, Costa Rica. |
| 37. | Ptilogonys caudatus, male, MNH no. 24492, 15 mi. SE Cartago, Costa Rica. |
| 38. | Phainoptila nitens, male, MNH no. 24754, Pima Co., Arizona. |
| 39. | Ptilogonys cinereus, female, Louisiana State University no. 297, Xilitla Region, San Luís Potosi, Mexico. |
| 40. | Dulus dominicus, female, USNM no. 292652, Don Don, Haiti. |
| 41. | Bombycilla cedrorum, male, MNH no. 15331, Bexar Co., Texas. |
| 42. | Bombycilla garrula, sex?, USNM no. 223895, Bozeman, Montana. |
In specialization for muscle attachment, the centra of the pygostyles
of the Ptilogonatinae have more area for muscle attachment
[Pg 505]
than do the centra in the Bombycillinae and Dulinae; the centrum
is wide, the spinous portion is long, and the bone is flattened anteriorly.
The most generalized pygostyle is in Phainoptila, and that
of Dulus differs only slightly. In Bombycilla the pygostyle is proportionately
small, but is complex in shape; there is seemingly not
the need for greatly expanded areas since the caudal muscles are
less specialized in this genus.
[↑ TOC]
Sternum.—The sternum in Bombycillids is typically passerine in
general shape and in having a long and deep carina or sternal crest.
The caudal process of the bone is broad, with the terminal ends
flattened, forming dorsally a graceful V-shaped outline, whereas
the outline of the posterior end of the sternum is broad and convex.
In lateral aspect, the carina is deeper in Bombycilla than in other
genera of the family, and is deepest in B. garrula. In this species, the
manubrium is more extended and comparatively larger than in the
other species of the family. The anterior edge of the keel forms
the sharpest angle in B. cedrorum. In Dulus, the keel is moderately
deep, the manubrium short, and there is a distinct indented curve
between the manubrium and the anterior angle of the keel.
In ventral aspect the lateral processes of the sternum tend to
flare outwards in adult Ptilogonatines on almost the same plane
as the rest of the bone, whereas in Bombycilla and Dulus the same
process is closer to the body of the sternum. In Bombycilla the
xiphoid process is more dorsal in position than in other species in the
family, and in Dulus an upward curve is very noticeable. The process
in these two genera is narrower than in the Ptilogonatinae, and
lacks the heavy distal terminal enlargement which is apparent in
Ptilogonys.
[↑ TOC]
Relative Lengths of Bones.—In instances where the animals
being compared are obviously different in over-all size, it is useful to
express the size of a given part in relation to some other part of
the same individual organism if the aim is to obtain clues as to
differences in functions of the parts being compared. Differences
in actual lengths of corresponding bones in two kinds of animals
often, of course, reflect only the difference in over-all size of the
animals. Consequently, the relative size of the part is expressed
as a percentage in this paper. In computing a percentage it is well,
of course, to select some relatively stable part of the animal to use as
a denominator in the mathematical expression that yields the percentage.
The thoracic region of the vertebral column is thought to
[Pg 506]
be such a part. For example, the length of the humerus divided by
the length of the thoracic region yields, in Phainopepla and Ptilogonys,
respective percentages of .84 and .85. These are roughly the
same, whereas the actual lengths of the humeri are 2.21 and 2.39 cm.
Table 4. Lengths of Leg Bones in cm.
| Species | Femur | Tibiotarsus | Tarsometatarsus |
| Ptilogonys caudatus | 2.04 | 3.10 | 1.94 |
| Ptilogonys cinereus | 1.89 | 2.90 | 1.77 |
| Phainopepla nitens | 1.76 | 2.78 | 1.72 |
| Phainoptila melanoxantha | 2.43 | 3.77 | 2.58 |
| Dulus dominicus | 2.09 | 3.34 | 2.09 |
| Bombycilla garrula | 2.32 | 3.46 | 1.99 |
| Bombycilla cedrorum | 1.92 | 2.95 | 1.64 |
Table 5. Leg-trunk Ratios (in percent)
| Species | Femur | Tibiotarsus | Tarsometatarsus | Total |
| Ptilogonys caudatus | 73 | 110 | 69 | 252 |
| Ptilogonys cinereus | 71 | 109 | 66 | 246 |
| Phainopepla nitens | 69 | 106 | 65 | 240 |
| Phainoptila melanoxantha | 74 | 115 | 60 | 249 |
| Dulus dominicus | 73 | 119 | 73 | 265 |
| Bombycilla garrula | 68 | 101 | 59 | 228 |
| Bombycilla cedrorum | 63 | 96 | 53 | 212 |
[Pg 507]
Table 6. Leg-trunk Ratios (in percent)
| Species | Femur | Tibiotarsus | Tarsometatarsus | Total |
| Corvus brachyrynchos | 71 | 120 | 77 | 268 |
| Corvus corax | 73 | 139 | 78 | 290 |
| Dendroica audubonii | 62 | 109 | 81 | 252 |
| Setophaga ruticilla | 66 | 127 | 94 | 287 |
| Myadestes townsendi | 61 | 99 | 60 | 220 |
| Sialia sialis | 66 | 111 | 72 | 249 |
| Hylocichla mustelina | 75 | 133 | 97 | 305 |
| Parus atricapillus | 78 | 138 | 99 | 315 |
| Tachycineta thalassina | 61 | 97 | 56 | 214 |
| Myiarchus crinitus | 68 | 106 | 74 | 248 |
| Dumetella carolinensis | 73 | 136 | 94 | 303 |
| Polioptila caerulea | 75 | 144 | 113 | 332 |
| Eremophila alpestris | 73 | 113 | 115 | 301 |
| Muscivora forficata | 62 | 98 | 61 | 221 |
Table 7. Actual Length and Width in mm. of Pygostyle and Proportionate
Length and Width of Pygostyle in percent of Lacrimal Length
| Species | Length | Width | Length, percent | Width, percent |
| Ptilogonys caudatus | 9.8 | 3.9 | 45 | 18 |
| Ptilogonys cinereus | 8.8 | 4.1 | 41 | 19 |
| Phainopepla nitens | 8.4 | 3.9 | 41 | 19 |
| Phainoptila melanoxantha | 8.5 | 3.5 | 35 | 14 |
| Dulus dominicus | 8.5 | 2.9 | 38 | 13 |
| Bombycilla garrula | 7.0 | 3.5 | 31 | 15 |
| Bombycilla cedrorum | 7.1 | 2.9 | 35 | 14 |
[Pg 508]
Table 8. Length of Sternum and Depth of Carina expressed as percentages
of the Length of the Trunk
| Species | Sternum | Carina |
| Ptilogonys caudatus | 85 | 28 |
| Ptilogonys cinereus | 91 | 32 |
| Phainopepla nitens | 81 | 26 |
| Phainoptila melanoxantha | 76 | 25 |
| Dulus dominicus | 107 | 28 |
| Bombycilla garrula | 88 | 33 |
| Bombycilla cedrorum | 82 | 31 |
Table 9. Skull and Sternum, Length and Width in mm.
| Species | Length of Skull | Width of Skull | Length of Sternum | Width of Sternum |
| Ptilogonys caudatus | 34.9 | 15.6 | 23.9 | 7.8 |
| Ptilogonys cinereus | 33.4 | 14.7 | 24.3 | 8.5 |
| Phainopepla nitens | 33.3 | 15.1 | 21.3 | 6.9 |
| Phainoptila melanoxantha | 39.7 | 16.0 | 24.8 | 8.2 |
| Dulus dominicus | 36.4 | 16.6 | 30.5 | 8.0 |
| Bombycilla garrula | 37.0 | 16.8 | 30.0 | 11.2 |
| Bombycilla cedrorum | 34.0 | 15.5 | 25.3 | 9.6 |
The length of the trunk was taken as the distance from the
anterior tip of the neural crest of the last cervical vertebra to the
anterior edge of an acetabulum. The number of free thoracic
vertebra was five in each specimen; consequently, there was no error
from this source. In the cranium, a measurement was taken from
the anterior edge of the lacrimal bone to the posteriormost end of the
cranium, and the resultant figure was employed for a constant in
cases in which small bones were compared.
[Pg 509]
Table 10. Relative Length and Width of Skull (in percent)
| Species | Length of Skull | Width of Skull | ||
| Ptilogonys caudatus | 160 | 72 | ||
| Ptilogonys cinereus | 158 | 69 | ||
| Phainopepla nitens | 162 | 73 | ||
| Phainoptila melanoxantha | 161 | 65 | ||
| Dulus dominicus | 164 | 75 | ||
| Bombycilla garrula | 164 | 74 | ||
| Bombycilla cedrorum | 162 | 74 | ||
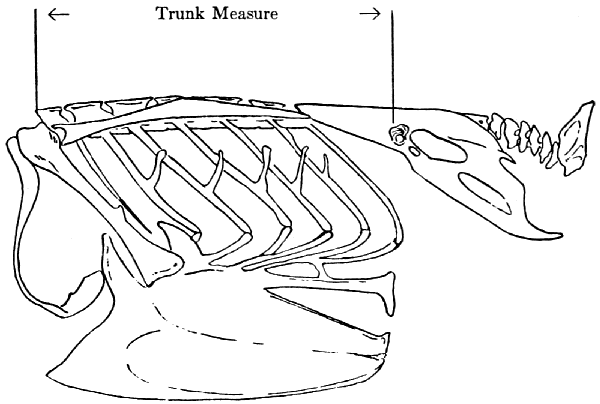
Fig. 43. Part of skeleton of Bombycilla cedrorum showing method of
measuring the length of the trunk. Natural size.
[↑ TOC]
Leg-trunk Percentages.—Table 4 shows the relative lengths of the
legs and of the separate bones in the legs of the different species of
the Bombycillids. Table 5 shows corresponding lengths for other
passerine birds. The total length of the leg was computed by adding
the figures obtained for the lengths of the femur, tibiotarsus and
[Pg 510]
tarsometatarsus. The lengths of the toes were disregarded. Length
of leg was recorded in this same way by Richardson (1942:333), who
thought that only in swimming and running birds do the toes contribute
to the functional length of the hind limb.
Table 4 shows that of the birds compared in this paper, Dulus
has the longest legs. In order of decreasing length the others are
the Ptilogonatinae, and finally the Bombycillinae, which have the
shortest legs of all. In Waxwings the length of the legs, expressed
as percentages of the body-lengths, are identical with those birds
that are similar in habits, that is to say, birds which do not use the
hind limb except in perching. It can be noted by reference to Table
5 that Tachycineta and Myadestes fall into this category. This
shortness of limb is obviously adaptive, and each of the segments of
the limb has been correspondingly shortened, with no element reduced
at the expense of the other two. The short leg can be more
easily folded against the body while the bird is in flight, than can a
long leg which is more unwieldy. It may be noted from tables 4 and
5 that birds which spend much time on the ground, or that hop a
great deal in the underbrush, have longer legs than do birds which
spend much time in flight. Two birds with noticeably long legs
are Hylocichla mustelina, a typical ground dweller, and Parus atricapillus,
which hops about in the trees and underbrush.
Insofar as the lengths of the legs show, Dulus and Phainoptila are
the most generalized of the Bombycillidae, since the relative length
of leg is approximately the same as that of more generalized birds
such as warblers, crows and thrushes of similar locomotory habits.
In other words, Dulus and Phainoptila have remained unspecialized,
in contrast to the waxwings in which adaptive changes fitting them
for a perching habit have taken place. Ptilogonys and Phainopepla
are intermediate in length of leg between Phainoptila and Bombycilla,
and Ptilogonys and Phainopepla have progressed from life on
the ground toward the perching habit. Bombycilla cedrorum is
more specialized than is B. garrula in shortness of leg, and the reduction
is comparable, as is noted above, to that in the legs of
Tachycineta.
In birds which have the legs much modified for walking or for hopping
in the brush, such as Polioptila and Eremophila, it is noteworthy
that the distal segment, the tarsometatarsus, is the longest, whereas
in birds such as Myiarchus and Tachycineta, that do not utilize the
limbs in this manner, the tibiotarsus, the middle segment, is the
longest. Mammals much modified for walking or hopping likewise
[Pg 511]
have the proximal segment, the femur, short, and the distal segment
long (Howell, 1944). The waxwings have all of the segments short;
these birds are modified for strong and sustained flight. Their hind
limbs are used principally for landing devices and for perching. No
one element of the leg has been shortened much, if any, more than
any other.
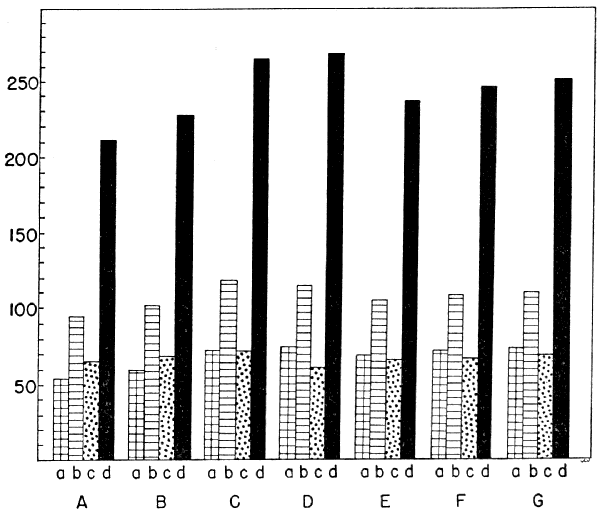
Fig. 44. Graph showing relative lengths of bones of the leg. The percentage
values are shown on the axis of the ordinates.
A. Bombycilla cedrorum; B. Bombycilla garrula; C. Dulus dominicus; D. Phainoptila melanoxantha; E. Phainopepla nitens; F. Ptilogonys cinereus; G. Ptilogonys caudatus.
a. femur; b. tibiotarsus; c. tarsometatarsus; d. total.
[↑ TOC]
Arm-trunk Percentages.—Tables 1 and 2 show the total length
of the arm, and lengths of the separate arm elements, relative to the
trunk. Table 3 gives the corresponding lengths for birds other than
the Bombycillidae. Total length of arm was obtained by adding
together the lengths of the humerus, ulna, and manus, and by dividing
the figure thus obtained by the length of the trunk as was
done for leg lengths in tables 4 and 5. The method of adding together
the component parts does not give the entire length of the
[Pg 512]
wing, since the length of the feathers, which add effectively to the
total length, as well as do the lengths of the small carpal elements,
is lacking.
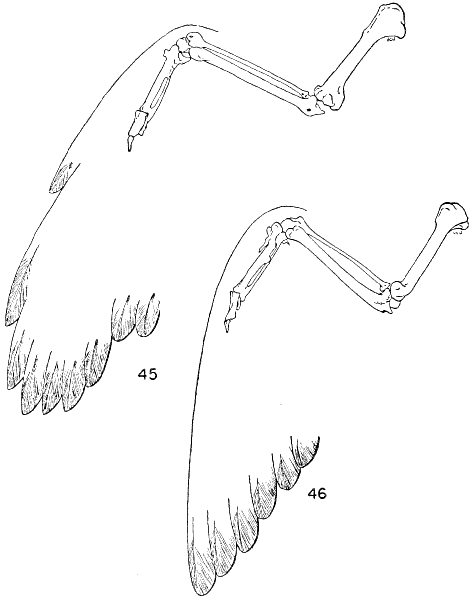
Figs. 45-46. Outlines of wings. × 1/2
45. Ptilogonys caudatus, showing relation of outline of wing to bones of arm.
46. Bombycilla cedrorum, showing relation of outline of wing to bones of arm.
It may be noted that Phainoptila and Bombycilla have the shortest
arm in the family Bombycillidae. The humerus, radius and ulna
are comparable to the same elements in thrushes and the catbird,
and it is only the extremely short manus in Phainoptila that affects
the total. The manus in Phainoptila is comparatively smaller than
in any other genus of the family Bombycillidae, and this indicates
poor flight power. Bombycilla has a total length corresponding
[Pg 513]
closely to that in warblers, but the lengths of the distal elements
correspond closely to those in the catbird and thrushes. Of the
three segments, the humerus is, relatively, the most shortened. Next
in order of increasing length of arm is Dulus; measurements for it
are roughly the same as those of Myadestes. The wing bones of the
Ptilogonatinae, other than Phainoptila, are the longest in this series,
and they most nearly resemble the same bones in flycatchers, Parids,
and gnatcatchers.
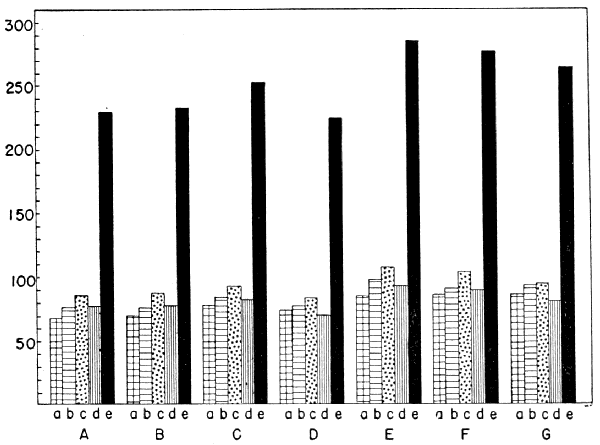
Fig. 47. Graph showing relative lengths of bones of the arm. The percentage values are shown on the axis of the ordinates.
A. Bombycilla cedrorum; B. Bombycilla garrula; C. Dulus dominicus; D. Phainoptila melanoxantha; E. Phainopepla nitens; F. Ptilogonys cinereus; G. Ptilogonys caudatus.
a. humerus; b. radius; c. ulna; d. manus; e. total.
It is notable that, in general, birds with long and narrow wings
appear to have relatively the shortest humeri, with the distal bones,
especially the manus, variable in length and seemingly correlated
with the manner of feather attachment. Those birds with rounded
and short wings have the longest humeri. In swallows, for example,
the humerus is short, whereas the other arm bones are long, and the
manus is unusually large and heavy. A short humerus gives better
lever action in the flight stroke than a long humerus does.
[↑ TOC]
[Pg 514]
Dissections showed the same muscles to be present in all genera
of the Bombycillidae. There are, nevertheless, differences in the
size of the muscles in the various species, and these differences have
been investigated primarily as a check on differences noted in the
structure of the bones. Even slight differences in mass can be important
functionally, but the difficulty in accurately measuring the
mass prevents wholly reliable conclusions. The method first used in
the attempt to determine the mass of a given muscle was that of
immersing the muscle in a liquid-filled graduated tube, and then
measuring the amount of liquid displaced. This method, although
adequate for large muscles, was subject to a great amount of error in
the case of small muscles, and consequently was abandoned. The
technique eventually used was that previously employed by Richardson
(1942). It consisted of dissecting out the muscle, placing it
in embalming solution, leaving it there until a later period, and
finally, weighing the muscle on scales, accurate to a milligram,
after the muscle had been out of the liquid for a period of one
minute. After being weighed, the muscle was measured by the displacement
method in a graduated tube, as a check. The results indicate
that, although the two methods give the same general results,
weighing is accurate to one-hundredth of a gram, whereas the displacement
method was accurate to only a tenth of a gram.
In determining the percentage of the weight of a muscle in relation
to the total weight of the bird, the weight of the muscle was used as
the numerator, and the weight of the preserved specimen was used
as the denominator. Before weights were taken, all specimens were
plucked in identical fashion.
[↑ TOC]
Caudal Muscles.—The muscles of the caudal area that were used
for comparison were the levator caudae and the lateralis caudae.
These muscles are used by the living bird to maintain the position of
the pygostyle and therefore the rectrices; these muscles are especially
important to those birds that utilize the tail as a rudder
in flight and as a brake. As may be seen by reference to Table
11, the two muscles are largest in proportion to body weight in the
Ptilogonatinae, in which subfamily the species have long rectrices
and must have correspondingly well-developed muscles in order to
utilize the rectrices to best advantage in flight. The lateralis caudae
differs more according to species than does the levator caudae,
showing that rudder action of the tail is of primary importance in the
adaptation for capturing insects. It will be remembered that the
[Pg 515]
pygostyle in this subfamily has a flattened lateral surface for attachment
of the levator caudae muscle, and it is therefore to be expected
that this muscle will be larger in the Ptilogonatinae than it is in
either the Bombycillinae or the Dulinae. The levator coccygis, together
with the two muscles mentioned above, is responsible for
elevation of the tail. The levator coccygis is less altered in different
species of the family than is the lateralis caudae. It may be noted
that the caudal muscles of Dulus and Bombycilla constitute a smaller
percentage of the total weight of the bird than in any of the genera
in the subfamily Ptilogonatinae.
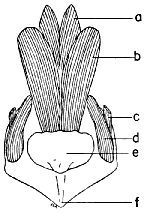
Fig. 48. Caudal musculature, of Phainopepla nitens lepida, in dorsal view. × 2.
a. Levator coccygis; b. Levator caudae; c. Lateralis caudae;
d. Lateralis coccygis; e. oil gland; f. dorsal tip of pygostyle.
Table 11. Caudal Muscles (Actual and Relative Weights)
| Species | Levator | Lateralis |
| Ptilogonys caudatus | .145g. | .022g. |
| .092% | .045% | |
| Ptilogonys cinereus | .030g. | .010g. |
| .076% | .026% | |
| Phainopepla nitens | .025g. | .008g. |
| .096% | .029% | |
| Phainoptila melanoxantha | .040g. | .015g. |
| .063% | .014% | |
| Dulus dominicus | .028g. | .006g. |
| .063% | .014% | |
| Bombycilla garrula | .034g. | .010g. |
| .048% | .014% | |
| Bombycilla cedrorum | .026g. | .008g. |
| .050% | .014% | |
[Pg 516]
Table 12. Weights of Muscles (These percentages expressed in terms of
weights of the body)
| Species | P. major | P. minor | Deltoid | Thigh | Peroneus | Gastrocnemius |
| Ptilogonys caudatus | 2.42g. | .29g. | .55g. | .43g. | .15g. | |
| 4.94% | .59% | 1.12% | .88% | .31% | .96% | |
| Ptilogonys cinereus | 2.19g. | .28g. | .53g. | .30g. | .08g. | |
| 5.57% | .71% | 1.35% | .71% | .21% | 1.02% | |
| Phainopepla nitens | 1.30g. | .20g. | .30g. | .28g. | .10g. | |
| 4.99% | .77% | 1.15% | 1.12% | .40% | 1.42% | |
| Phainoptila melanoxantha | 3.93g. | .44g. | .92g. | 1.09g. | .48g. | |
| 6.18% | .69% | 1.45% | 1.61% | .75% | 2.97% | |
| Dulus dominicus | 2.09g. | .22g. | .50g. | .73g. | .18g. | |
| 4.81% | .50% | 1.15% | 1.68% | .41% | 1.01% | |
| Bombycilla garrula | 3.85g. | .45g. | .55g. | .50g. | .15g. | |
| 5.31% | .62% | .76% | .69% | .18% | .59% | |
| Bombycilla cedrorum | 2.58g. | .35g. | .50g. | .37g. | .10g. | |
| 5.00% | .68% | .97% | .73% | .19% | .83% | |
[↑ TOC]
[Pg 517]
Pectoral Muscles.—The pectoral set of muscles varies but little in
the family; flight power is seemingly not dependent upon size of
either the pectoralis major or pectoralis minor. The data indicate
that the insertion on the humerus, with consequent changes in the
relative length of that bone, is more significant in type of flight and
over-all flight power than is the actual size of the muscle mass. The
deltoid muscle, for example, is smaller in Bombycilla than in members
of the other two subfamilies. The humerus in Bombycilla is
shortened, and the muscle therefore does not need to be large to
accomplish the same powerful stroke that would be accomplished
by a longer humerus and a larger, more powerful deltoid muscle.
In the case of the deltoid, the shortening of the humerus and the
more complex arrangement of the points of insertion have obviated
the necessity of enlarging the muscle.
[↑ TOC]
Leg Musculature.—The muscles of the thigh are noticeably larger
in birds that have long leg bones. (See Table 12 for size of muscles.)
On the tibiotarsus, the peroneus and gastrocnemius muscles
were measured. When expressed as a percentage of the weight of
the bird, the peroneus has much the same relative weight in all but
one of the species, whereas the gastrocnemius varies much. The
peroneus is proportionately large only in Phainoptila, in which
genus all the leg muscles are well developed, but the gastrocnemius
is larger in all the Ptilogonatinae and in Dulus than it is in the
specialized Bombycilla, in which it has probably been reduced as
the leg bones and other muscles have been reduced.
The volume of the muscles of the hind limb changes more readily
in response to saltation and running than do the muscles of the
forelimb to flying.
The digestive tract is relatively uniform in all genera of the family;
there are only slight differences between the species. The
degree of compactness of the visceral mass varies, Phainoptila and
Ptilogonys caudatus having the folds of the digestive tract loosely
arranged, whereas Ptilogonys cinereus and Phainopepla have folds
which adhere more tightly to the ventriculus and liver. In Dulus
and Bombycilla, as compared with the Ptilogonatinae, the visceral
mass (primarily liver and ventriculus) is situated more posteriorly
in the body cavity, and is more compact, and the intestine is more
tightly coiled.
The coiling of the intestine, if its degree of compactness is disregarded,
is nearly identical in the birds of the family; there are
[Pg 518]
four major loops between the ventriculus and the anus. The length
of this section of the tract is, however, somewhat variable, as can be
seen by reference to Table 13, in which the actual and relative
lengths of the intestine are given. It may be seen that in Bombycilla
and in Phainopepla, the tracts are much shortened. This is
notable, since these are frugivorous birds, and in many frugivorous
birds, the tract is lengthened for better extraction of edible portions
of the food. Possibly the action of the digestive juices is correspondingly
more rapid in Bombycilla and Phainopepla, thereby permitting
the necessary nutriment to be extracted by a short digestive
tract.
In a migratory bird, or one that depends on flight power to find
food and escape capture by predators, as in the case of the waxwings,
the compacted and shortened visceral mass would seem to be
advantageous, because of the consequent reduction in weight. I
consider the longer intestine to be the ancestral condition, and that
the intestine has become shorter to meet new environmental conditions.
Table 13. Digestive Tract: Actual Length, and Length Relative to
Thoracic Length
| Species | Length in mm. | Relative length (in percent) |
| Ptilogonys caudatus | 134 | 476.9 |
| Ptilogonys cinereus | 111 | 415.6 |
| Phainopepla nitens | 94 | 357.5 |
| Phainoptila melanoxantha | 150 | 457.1 |
| Dulus dominicus | 130 | 451.0 |
| Bombycilla garrula | 102 | 298.2 |
| Bombycilla cedrorum | 95 | 309.5 |
Beddard (1898:30) states that caecae in the tract may be highly
variable in a single family of birds. The Bombycillidae is no
exception in this regard. At the junction of the cloaca and the large
intestine, there are two small caecae, the function of which is unknown
to me. The caecae are largest in the Ptilogonatinae, smaller
in the Bombycillinae, and smallest in the Dulinae. There may be a
[Pg 519]
correlation between large caecae and more insectivorous diet and
small caecae and frugivorous diet; however, the data are not conclusive
in this regard.
It is here postulated that the center of origin for the ancestral
stock of the Bombycillidae was in a region of North America, which
at the time concerned was temperate or possibly even semi-tropical
in climate. Probably Northern Mexico was the place and probably
the climate was temperate. It is reasonably certain, because of the
distribution of the species of the family, that they originated in the
Americas. In the absence of paleontological data (Bombycilla alone
is reported, in essentially its modern form, from the late Pleistocene—Wetmore,
1940a), the place and time of origin cannot certainly be
determined.
The distribution of the family is such that the more primitive
groups are in the south. These are the Ptilogonatinae in Central
America and Mexico, and the isolated Dulinae in Haiti and the
Dominican Republic. This distribution would support the view
that the origin was in the south. However, the Holarctic Bombycillinae
are so typically birds of northern latitudes that, were it
not for such close relatives south of their range, it would appear
logical to infer a northerly origin with a subsequent shifting of
populations both southward and northward. The phyletic age of the
family is probably great, however, as evidenced by the spotty distribution
of the birds.
In the evolution of this family, population pressure possibly
played the initial role in forcing members of the primitive, southern
stock to seek habitable areas on the periphery of the range. Some
birds also, being possessed of the "adventuresome spirit", aided the
northerly movement, thus effecting an extension of the breeding
ranges to the north. So far as is now known, this family did not
seek living space in South America. By extending its range, a species
might find more abundant food and nesting sites. This process
of extending the range probably would be costly to the species concerned,
because only those individuals best able to adapt themselves
to the new environmental conditions would be able to survive long
enough to reproduce their kind.
The return flight to the south could, in time, be dispensed with,
except in the coldest weather or when the local berry- and fruit-crop
failed. Birds such as waxwings are, of course, able to subsist on
[Pg 520]
dried fruits and berries in the critical winter season when strictly
insectivorous birds, not so catholic in their food habits, must return
south. It appears that waxwings are descendants of migratory birds
that have adjusted themselves to a life in the north; and they are
judged not to have evolved from year-round residents of the north.
Even a short migratory journey in spring by part of a population
of birds, while the other part remained in the original range, would
quickly isolate one breeding population from the other, resulting in
the formation of different genetic strains that lead to subspecies,
species, and finally to genera and families. Any variation away
from the ancestral, "sedentary" stock would become established
more quickly because of such isolation at the breeding period. By
the same token, the parental stock can, and no doubt does, become
modified to suit its environment more perfectly, thus accelerating
the tempo of this type of divergent evolution.
The original "split" of the Bombycillines is thought then to have
been the result of migration on the part of some of the ancestral
stock, with subsequent loss of regular migration because the need
to return south was lost. Early in development, and before the migrational
tendency was entirely lost, an isolated population, which
later became sedentary, as it was an island population, diverged to
give rise to the Dulinae. The Dulinae are a homogeneous group
since on the islands now inhabited by the birds, they have not been
isolated sufficiently long to produce even well-marked subspecies.
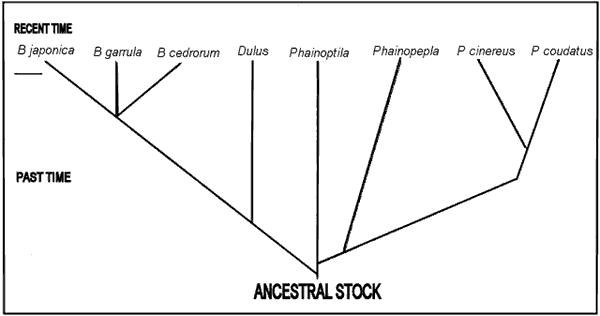
Fig. 49. Hypothetical family tree of the Bombycillidae.
The present day Phainoptila is most nearly like the ancestral
group, and the remainder of the Ptilogonatinae have diverged to
[Pg 521]
fit conditions similar to those to which the Tyrannid flycatchers,
which parallel them, are also fitted.
In comparatively recent geological time, two basic lines developed
from the Bombycilline stock, the future B. garrula and B. cedrorum.
Possibly garrula originally was isolated in Europe and Asia, and
later came into contact with B. cedrorum, following the time at
which the two species were genetically well differentiated. It
appears certain that B. japonica was an offshoot of the Bombycilline
stock at an early time, since it has characteristics that seem relatively
unspecialized. It possibly was isolated in the Orient.
Structural affinities of Dulus and Bombycilla are more pronounced
than are those of Dulus and Ptilogonys, for example. Many of the
structural features of Dulus parallel those of Phainoptila, and it
seems likely that the Dulinae were separated early in the history of
the family, perhaps as an isolated offshoot of the early migratory
Bombycillinae.
Nomenclature, as used by a taxonomist, should of course indicate
affinities as well as apply a name, and the rank of the family should
be applied to a structural unit based on common anatomical characters
that are more fundamental than, in my opinion, are those
used by Ridgway (1904) in proposing family status for the silky
flycatchers and the palm-chats. The characters in the diagnosis
(page 478) of the family Bombycillidae are common features regarded
as warranting a single family unit for the waxwings, silky
flycatchers, and palm-chats. The differences in morphology used by
previous workers to characterize each of these groups: (1) the silky
flycatchers; (2) waxwings and; (3) palm-chats are regarded as more
properly characters of only subfamily rank.
The existing coloration of the species of the Bombycillidae appears
to have been acquired relatively late, geologically speaking.
The three subfamilies responded to ecological stimuli in three different
ways, and the resulting color patterns are unlike in the three
groups. Dulinae to this day have a color pattern that is most like
the ancestral color pattern, and this is recapitulated in the juvenal
plumage of the Bombycillinae before they attain their adult plumage.
Consideration of the geographic distribution of the species of the
family indicates that the center of origin of the family Bombycillidae
[Pg 522]
was south of the present range of the waxwings (subfamily
Bombycillinae). Waxwings probably are the descendants of a migratory
population that diverged from the primitive population at
an early time in the history of the family. Owing to their adaptations
to survive in the north, waxwings no longer return south in
the autumn. Palm-chats (subfamily Dulinae) are descendants of
an isolated population of the family stock that developed communal
living habits as one specialization. Silky Flycatchers (subfamily
Ptilogonatinae) became modified to catch insects, and have specializations
that roughly parallel those of the Tyrannid flycatchers.
Osteologically, the various species of the Bombycillidae are remarkably
similar. Small variations do exist, but these are primarily
differences in relative size. The modifications of the beak
enable palm-chats to feed on parts of plants, and the beak of
Phainoptila shows some similarity in this respect. Rounded wings,
which cause a bird to fly by means of short, relatively weak strokes,
are correlated with a comparatively long humerus, whereas long and
pointed wings, which enable a bird to fly with more powerful strokes
of the wing, are correlated with a relatively short humerus. There
is a positive correlation between a short humerus and a long external
condyle, and between a long humerus and the absence or
smallness of the external condyle.
In the Bombycillidae short bones of the leg are adaptive, and
long bones of the leg are the generalized condition. Although all
passerine birds were differentiated relatively late in geologic time,
long hind limbs still could have been present in the immediate ancestors
of passerine birds. As adaptive radiation took place in the
class Aves, some birds, the Bombycillidae included, became more
and more adapted for an arboreal, and eventually an aerial habitat,
with consequent loss of saltatorial and running ability.
Birds, like mammals, have a short femur, the most proximal element
in the leg, if the species is adapted to run fast. If the species
is not adapted to run fast, birds, unlike mammals, have the tibiotarsus
longer than any of the other elements; in mammals that are
not adapted to run fast, the femur and tibia are approximately the
same length. In non-running birds as compared with running birds,
the leg element distal to the tibiotarsus, and the one proximal to it,
are considerably shortened. In waxwings, all three elements of
the hind limb are shortened, indicating that the reduction in length
has been, evolutionarily speaking, a rapid process, in order to reduce
the limbs to a convenient size as soon as possible.
[Pg 523]
The shape of the pygostyle varies in the Bombycillidae, but the
simple shieldlike bone of Phainoptila is judged to resemble closely
the ancestral type. In Ptilogonys there is a tall dorsal spine,
coupled with a wide and heavy centrum and flattened lateral areas,
for support of the long rectrices. In Bombycilla the bone is small
with knobs on the centrum that have been developed for muscle
attachment.
The muscles were carefully dissected in each genus and in most
of the species. The same homologous muscles are present in all
species. Significant differences were found only in the relative size
of certain muscles. No satisfactorily accurate method of measuring
these differences was found. Consequently, less use was made of
the results of the dissections than was originally planned.
The set of pectoral muscles varies but slightly in relative mass,
and the variation is not considered significant. The deltoid muscle
was selected for measurement since its point of insertion is unusually
variable, while the mass of the muscle varies little. We can conclude
that the extent of the area of insertion of the tendon of a
muscle can determine that muscle's relative efficiency, while the
muscle itself remains the same in bulk.
The muscles of the hind limb are notably larger in species that
have long legs, and a good index of the hopping ability may be
gained by study of certain of these muscles. In the Bombycillidae,
and in those Ptilogonatinae that do not use the hind limbs for
hopping, the bones are shortened, and the associated muscles are
correspondingly smaller.
The gross anatomy of the digestive tract is practically identical
in the members of the family. The variability noted is mainly in
the degree of compactness of the visceral mass in Bombycilla and
in Phainopepla. Also there is a tendency for the Bombycillinae
and the Dulinae to have the mass situated more posteriorly than it
is in the Ptilogonatinae. Moreover, Bombycilla has a shorter intestine
than do the other genera. All of this indicates that the waxwings
(Bombycillinae) have the center of gravity situated more
advantageously for flight than do the birds of the two other subfamilies.
[Pg 524]
| 1. | The silky flycatchers, waxwings, and palm-chats are included in the family Bombycillidae; the Ptilogonatidae and Dulidae are reduced to subfamily rank. |
| 2. | The coloration of the birds of each subfamily is different because the ecological needs are different. |
| 3. | Waxwings were at one time regularly migratory, but are now nomadic, since they are adapted to live in northern latitudes for the entire year. |
| 4. | The corresponding bones in different members of the family closely resemble one another, and the differences which do exist are the results of responses within relatively recent times to changes in habits. |
| 5. | In the Bombycillidae a rounded wing is judged to be the primitive condition. As the wing becomes more pointed, the humerus becomes shorter and its external condyle longer. |
| 6. | The hind limbs are short in birds that depend most on flight power, but are longer and the distal elements are disproportionately longer in birds that depend on saltation or on running. |
| 7. | The pygostyle varies in shape and size between genera and even between some species. |
| 8. | The pectoral muscles differ in size only slightly in the different members of the family, but the insertions are more extensive for these muscles in birds that fly a great deal. |
| 9. | The muscles of the hind limb vary in mass, but not in kind, in the members of the family Bombycillidae. |
| 10. | In the Bombycillidae that depend on flight power, rather than on saltation or on running power, there is a tendency for the digestive tract to become shorter and for the whole visceral mass to become more compact. |
[Pg 525]
1915. Nesting of the Bohemian Waxwing in northern British Columbia. Condor, 17(4):145-148, 1915.
1907. A collecting trip in Korea. Condor, 9(5):146-147, 1907.
1909. Nesting of the Bohemian Waxwing (Bombycilla garrulus). Auk, 26(1):10-12, 1909.
1942. Bird display. Cambridge Univ. Press, xvi + 381 pp., 22 plates, 1942.
1860. The birds of North America. J. B. Lippincott Co., lvi + 1003 pp., 1860.
1898. The structure and classification of birds. Longmans, Green & Co., xx + 548 pp., 252 figs., 1898.
1917a. A study of the incubation period of birds. Kendrick-Bellamy Co., 109 pp., 1917.
1917b. Regurgitation in the Bohemian Waxwing. Auk, 34(3):341-342, 1917.
1924. A summer occurrence of the Bohemian Waxwing in Colorado. Auk, 41(4):614, 1924.
1926. Remarks on the origin and distribution of the Zonotrichiae. Auk,
18(3):326-332, 1926.
1921. Breeding birds of Warland, Lincoln County, Montana. Auk, 38(4):552-565, 1921.
1930. Adaptive modifications in the woodpeckers. Univ. California Publ.
Zoöl., 32(8):455-524, 29 figs. in text, 1930.
1909-1912. An annotated list of the birds of Costa Rica including Cocos
Island. Ann. Carnegie Mus., 6(1):314-915, 1909-1912.
1886. The birds of the West Indies, etc. Auk, 3(2):187-245, 1886.
1936. Nesting habits of the Cedar Waxwing. Auk, 53(1):1-8, 1936.
1943. Distribution and habitat relationships of the Phainopepla. Auk,
60(3):319-333, 1943.
1938. Cursorial adaptations in birds—limb proportions in the skeleton of
Geococcyx. Jour. Morph., 63:207-217, 3 figs. in text, 1938.
1940. Structural adaptations in Thrashers (Mimidae: Genus Toxostoma)
with comments on interspecific relationships. Univ. California Publ.
Zoöl., 42(7):341-400, 24 figs. in text, 1940.
[Pg 526]
1924. Abnormal Cedar Waxwing. Auk, 41(1):160, 1924.
1946. Adaptations and comparative anatomy of the locomotor apparatus of New World Vultures. Amer. Midl. Nat., 35:545-727, 14 plates, 42 tables, 28 figs. in text, 1946.
1939. Die Färbung der Vogelfeder durch Pigment und Struktur. Jour. für Orn., 87:426-523, 1939.
1876. On some anatomical peculiarities which bear upon the major divisions of the passerine birds, Pt. I. Proc. Zoöl. Soc. London, 626-647, 1876.
1948. Le jaseur boreal en Suisse pendant l'hiver 1946-1947. Der Orn. Beob., 45(1):1-5, 1948.
1862. The birds of Great Britain. London, published by the author, 5 vols., text unpaged, 367 plates, 1862.
1901. The status of the Cedar Waxwing in California. Condor, 3(6):146-147, 1901.
1909. A new cowbird of the genus Molothrus. Univ. California Publ. Zoöl., 5:275-281, 6 figs. in text, 1909.
1934. The ornithology of Guerrero, Mexico. Mus. Comp. Zoöl. Bull. 75:367-422, 1934.
1933. A late nesting waxwing in central New York. Auk, 50(2):114-115, 1933.
1931. Nesting of the Bohemian Waxwing in British Columbia. Condor, 33(6):253-254, 1 fig., 1931.
1924. Die Vögel Mitteleuropas. Berlin, Huge Bermühler, 1:51-58, 1924.
1935. Catalogue of the birds of the Americas. Field Mus. Nat. Hist. Mus. Publ. 347, 8(pt. 8):vi + 541 pp., 1935.
1938. Muscles of the avian hip and thigh. Auk, 55(1):71-81, 2 figs. in text, 1938.
1944. Speed in animals, their specialization for running and leaping. Univ. Chicago Press, xi + 270 pp., 55 figs. 1944.
1937. Studies on the muscles of the pelvic appendage in birds. Amer. Midl. Nat., 18:1-108, 26 plates, 1937.
1948. Studies on the muscles of the pelvic appendages in birds II, the heterogeneous order Falconiformes. Amer. Midl. Nat., 39(1):102-127, 1948.
1909. Birds of the world. Henry Holt & Co., Ltd., xi + 873 pp., 15 plates, 233 figs. in text, 1909.
1943. A dictionary of animals. Tokyo, 3 + 767 + 50 pp., profusely illustrated, 1943.
1930. Ein Beitrag zur Kenntnis der Okologie, Biologie, und Geographie des Zobels (Martes zibellina L.). Zeits. für Okol. der Tierre, 19(2):291-320, 2 maps, 1930.
1928. Variations in the Fox Sparrow (Passerella iliaca) with reference to natural history and osteology. Univ. California Publ. Zoöl., 30(12):251-392, 4 plates, 38 figs. in text, 1928.
1928. History of a Cedar Waxwing family. Bull. NE Bird-Band. Assoc., 4:85-89, 1928.
1897. The tongues of birds. U. S. Nat. Mus. Report for 1895, 1001-1019 pp., 2 plates, 1897.
1906. Notes on birds observed while traveling from Yokohama to Manila. Condor, 8(4):98-100, 1906.
1939. Climate and evolution. N. Y. Acad. Sci., Spec. Publ., 1:xi + 223 pp., 1939.
1942. Systematics and the origin of species. Columbia Univ. Press, xiv + 334 pp., 29 figs. in text, 1942.
1947. Ecological factors in speciation. Evolution, 1(4):263-288, 1947.
1896. Nesting habits of Phainopepla nitens in California. Auk, 8(1):38-43, 1896.
1933. Postjuvenal molt and the appearance of sexual characters of plumage in Phainopepla nitens. Univ. California Publ. Zoöl., 38(13):425-444 pp., 8 pls., 1 fig. in text, 1933.
1937. Structural modifications in the Hawaiian Goose (Nesochen sandvicensis). A study in adaptive evolution. Univ. California Publ. Zoöl., 42(1):1-80, 6 plates, 12 figs. in text, 1937.
1941. Speciation in the avian genus Junco. Univ. California Publ. Zoöl., 44(3):173-434, 33 figs. in text, 1941.
1915. A northern winter record of the Phainopepla. Condor, 17(3):129, 1915.
1907. Nesting habits of Phainopepla nitens. Condor, 9(4):101-103, 1907.
1908. Observations on the nesting habits of Phainopepla. Condor, 10(2):72-75, 1908.
[Pg 528]
1909. Notes on the habits of Phainopepla nitens. Condor, 11(1):22-23, 1909.
1893-1896. A dictionary of birds. Adams and Charles Black, xii + 1086 pp., 1893-1896.
1940. Observations on the behavior of a young Cedar Waxwing. Condor, 43(1):58-64, 1940.
1917. A synopsis of the races of Bombycilla garrula (Linnaeus). Auk, 34(3):330-333, 1917.
1908. Northern range of the Phainopepla. Condor, 10(6):238, 1908.
1933. Molt of the Nonpareil. Auk, 50(2):121, 1933.
1916. The Cedar Waxwing (Bombycilla cedrorum) during July and August, 1916. Wilson Bull., 28:175-193, 1916.
1943. Breeding notes on Phainopepla. Auk, 60(3):333-341, 1943.
1942. Adaptive modifications for trunk foraging in birds. Univ. California Pub. Zoöl., 46(4):317-368, 2 plates, 16 figs. in text, 1942.
1904. The birds of North and Middle America, Part III. U. S. Nat. Mus. Bull. 50:xx + 801 pp., 19 plates, 1904.
1911. A study of the nesting of the Cedar Waxwing. Auk, 28(3):323-329, 1911.
1912. The probable breeding of the Bohemian Waxwing in Montana. Condor, 14(6):224, 1912.
1885. Catalogue of the birds in the British Museum, Vol. 10, British Mus., xiii + 682 pp., 12 plates, 1885.
1944. A flock of Cedar Waxwings meets tragedy. Condor, 46(4):205-206, 1944.
1887. A review of the muscles used in the classification of birds. Jour. Comp. Med. and Sur., 8(4):321-344, 1887.
1889a. Comparative osteology of the families of North American birds. Jour. Morph., 3(1):81-114, 6 plates, 1889.
1889b. Studies on the Macrochires, morphological and otherwise, with the view of indicating their relationships and defining their several positions in the system. Linn. Soc. London, Jour., 20(122):299-394, 1889.
1890. The myology of the Raven. Macmillan & Co., x + 344 pp., 76 figs., 1890.
1909. Osteology of birds. New York State Mus. Bull., 130:381 pp., 1909.
[Pg 529]
Manuscript—unpublished notes and personal correspondence.
1882. On the plumage of the waxwing, Ampelis garrulus, Linnaeus, from the examination and comparison of a large series of specimens killed, in Norfolk, in the winter of 1866-'67. Trans. Norfolk and Norwick Naturalists' Soc., 3:326-344, 2 figs. in text, 1882.
1940. Birds of Las Vigas, Veracruz. Auk, 57(2):234-243, 1940.
1942. Birds recorded in the Federal District and States of Puebla and Mexico by the 1939 Semple Expedition. Auk, 59(3):418-423, 1942.
1922. Birds and mammals of the Stikine River region of northern British Columbia and southeastern Alaska. Univ. California Publ. Zoöl., 24(2):125-314, 8 plates, 34 figs. in text, 1922.
1918. Bohemian Waxwing (Bombycilla garrulus) breeding within the United States. Auk, 35(2):226-227, 1918.
1934. Birds of Canada. Nat. Mus. Canada Bull., 72, series 19, 445 pp., 77 plates, 488 figs. in text, 1934.
1924. A remarkable Cedar Waxwing. Auk, 41(3):485, 1924.
1926. The migrations of birds. Cambridge, Harvard Univ. Press, vii + 217 pp., 1926.
1932. Notes from Dr. R. Ciferri on the birds of Hispaniola. Auk, 49(1):101-108, 1931.
1940a. A check-list of the fossil birds of North America. Smithson. Misc. Coll., 99(4):1-88 pp., 1940.
1940b. A systematic classification of the birds of the world. Smithson. Misc. Coll., 99(7):1-11 pp., 1940.
1931. The birds of Haiti and the Dominican Republic. U. S. Nat. Mus. Bull. 155:iv + 482 pp., 26 plates, 1931.
1905. Regurgitation feeding of nestlings. Auk, 22(1):54-71, 1905.
1928. The biography of a Cedar Waxwing. Bull. NE Bird-Band. Assoc., 4:77-85, 1928.
1945. The role of the pituitary, fat deposition, and body weight in bird migration. Condor, 47(3):95-127, 1945.
1857. On the nest and eggs of the Waxwing (Bombycilla garrula Tamm.). Proc. Zoöl. Soc. London, 25:55-56, 1857.
[Pg 530]
Mention should be made here of an important paper by Jean Delacour and
Dean Amadon (1949). The Relationships of Hypocolius (Ibis, 91:427-429,
plates 19 and 20) which appeared after the present paper by Arvey was
written. Delacour and Amadon stated that Hypocolius, a monotypic Persian
genus, should be assigned to the Bombycillidae. Their conclusions (op. cit.:429)
were as follows: "It might be advisable to set up three subfamilies in the
Bombycillidae, one for Bombycilla, one for Hypocolius, and a third for the
silky flycatchers, Ptilogonys, Phainopepla and Phainoptila. Further study may
show that Dulus can be added as a fourth subfamily.
"Previously the Bombycillidae appeared to be an American group of which
one genus (Bombycilla) had reached the Old World. Inclusion of Hypocolius
in the family makes this theory uncertain. Without obvious affinities to other
families, and consisting of a small number of scattered and rather divergent
genera, the Bombycillidae would seem to be a declining group whose origin
cannot safely be deduced from the distribution of the few existing species."
23-1019
[Pub i]
The University of Kansas Publications, Museum of Natural History, are
offered in exchange for the publications of learned societies and
institutions, universities and libraries. For exchanges and
information, address the Exchange Desk, University of Kansas Library,
Lawrence, Kansas, U. S. A.
Museum of Natural History.—E. Raymond Hall, Chairman, Editorial Committee.
This series contains contributions from the Museum of Natural History.
Cited as Univ. Kans. Publ., Mus. Nat. Hist.
| Vol. 1. | 1. | The pocket gophers (genus Thomomys) of Utah. By Stephen D. Durrant. Pp. 1-82, 1 figure in text. August 15, 1946. |
| 2. | The systematic status of Eumeces pluvialis Cope, and noteworthy records of other amphibians and reptiles from Kansas and Oklahoma. By Hobart M. Smith. Pp. 85-89. August 15, 1946. | |
| 3. | The tadpoles of Bufo cognatus Say. By Hobart M. Smith. Pp. 93-96, 1 figure in text. August 15, 1946. | |
| 4. | Hybridization between two species of garter snakes. By Hobart M. Smith. Pp. 97-100. August 15, 1946. | |
| 5. | Selected records of reptiles and amphibians from Kansas. By John Breukelman and Hobart M. Smith. Pp. 101-112. August 15, 1946. | |
| 6. | Kyphosis and other variations in soft-shelled turtles. By Hobart M. Smith. Pp. 117-124. July 7, 1947. | |
| 7. | Natural history of the prairie vole (Mammalian genus Microtus). By E. W. Jameson, Jr. Pp. 125-151, 4 figures in text. October 6, 1947. | |
| 8. | The postnatal development of two broods of great horned owls (Bubo virginianus). By Donald F. Hoffmeister and Henry W. Setzer. Pp. 157-173, 5 figures in text. October 6, 1947. | |
| 9. | Additions to the list of the birds of Louisiana. By George H. Lowery, Jr. Pp. 177-192. November 7, 1947. | |
| 10. | A check-list of the birds of Idaho. By M. Dale Arvey. Pp. 193-216. November 29, 1947. | |
| 11. | Subspeciation in pocket gophers of Kansas. By Bernardo Villa-R. and E. Raymond Hall. Pp. 217-236, 2 figures in text. November 29, 1947. | |
| 12. | A new bat (genus Myotis) from Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 237-244, 6 figures in text. December 10, 1947. | |
| 13. | Tadarida femorosacca (Merriam) in Tamaulipas, Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 245-248, 1 figure in text. December 10, 1947. | |
| 14. | A new pocket gopher (Thomomys) and a new spiny pocket mouse (Liomys) from Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa-R. Pp. 249-256, 6 figures in text. July 26, 1948. | |
| [Pub ii] | 15. | A new hylid frog from eastern Mexico. By Edward H. Taylor. Pp. 257-264, 1 figure in text. August 16, 1948. |
| 16. | A new extinct emydid turtle from the Lower Pliocene of Oklahoma. By Edwin C. Galbreath. Pp. 265-280, 1 plate. August 16, 1948. | |
| 17. | Pliocene and Pleistocene records of fossil turtles from western Kansas and Oklahoma. By Edwin C. Galbreath. Pp. 281-284, 1 figure in text. August 16, 1948. | |
| 18. | A new species of heteromyid rodent from the Middle Oligocene of northeastern Colorado with remarks on the skull. By Edwin C. Galbreath. Pp. 285-300, 2 plates. August 16, 1948. | |
| 19. | Speciation in the Brazilian spiny rats (genus Proechimys, Family Echimyidae). By João Moojen. Pp. 301-406, 140 figures in text. December 10, 1948. | |
| 20. | Three new beavers from Utah. By Stephen D. Durrant and Harold S. Crane. Pp. 407-417, 7 figures in text. December 24, 1948. | |
| 21. | Two new meadow mice from Michoacán, México. By E. Raymond Hall. Pp. 423-427, 6 figures in text. December 24, 1948. | |
| 22. | An annotated check list of the mammals of Michoacán, México. By E. Raymond Hall and Bernardo Villa R. Pp. 431-472, 5 figures in text. December 27, 1949. | |
| 23. | Subspeciation in the kangaroo rat, Dipodomys ordii. By Henry W. Setzer. Pp. 473-573, 27 figures in text, 7 tables. December 27, 1949. | |
| 24. | Geographic range of the hooded skunk, Mephitis macroura, with description of a new subspecies from Mexico. By E. Raymond Hall and Walter W. Dalquest. Pp. 575-580, 1 figure in text. January 20, 1950. | |
| 25. | Pipistrellus cinnamomeus Miller 1902 referred to the genus Myotis. By E. Raymond Hall and Walter W. Dalquest. Pp. 581-590, 5 figures in text. January 20, 1950. | |
| 26. | A synopsis of the American bats of the genus Pipistrellus. By E. Raymond Hall and Walter W. Dalquest. Pp. 591-602, 1 figure in text. January 20, 1950. | |
| Index. Pp. 605-638. | ||
| Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | 1. | The Avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| 2. | A Quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 46 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied species. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
Featured Books

The Wood Beyond the World
William Morris
is lot, whereas he had fallen into the toils of love of a woman exceeding fair, and had taken her to...

Bees from British Guiana
Theodore D. A. Cockerell
arked K. occur at Kalacoon.The body, or some part of it, brilliant greenNo part of the body brillian...

Comments on the Taxonomy and Geographic Distribution of Some North American Rodents
E. Raymond Hall and Keith R. Kelson
ens. Results of ourexamination are given below.Our studies have been aided by a contract (NR 161-791...

The Confessions of a Poacher
F.L.S. John Watson
hat was permanently attracted by hispersonality. Although eighty years of age there is stillsome of ...

Through Forest and Fire
Edward Sylvester Ellis
APTER XXVII.—WAS IT A JOKE?CHAPTER XXVIII.—THE TRAIL OF THE BEARCHAPTER XXIX.—HELP! HELP!CHAPT...

Chester Rand; or, The New Path to Fortune
Jr. Horatio Alger
RCHESTER IS DISCHARGEDINTRODUCES MR. SHARPLEIGH, THE DETECTIVECHESTER MEETS ANOTHER ARTISTA STRANGER...

Stories by Foreign Authors: Spanish
lid black; /* a thin black line border.. */ padding: 6px; /* ..spaced a bit out from the gr...

The Crystal Crypt
Philip K. Dick
""I don't blame them for giving us onelast going over," a heavy-set business mansaid to his companio...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Phylogeny of the Waxwings and Allied Birds" :