The Project Gutenberg eBook of Natural History of the Bell Vireo, Vireo bellii Audubon
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Natural History of the Bell Vireo, Vireo bellii Audubon
Author: Jon C. Barlow
Release date: June 17, 2010 [eBook #32855]
Language: English
Credits: Produced by Chris Curnow, Joseph Cooper and the Online
Distributed Proofreading Team at https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK NATURAL HISTORY OF THE BELL VIREO, VIREO BELLII AUDUBON ***
University of Kansas Publications
Museum of Natural History
Volume 12, No. 5, pp. 241-296, 6 figs.
March 7, 1962
Natural History of the Bell Vireo,
Vireo bellii Audubon
BY
JON C. BARLOW
University of Kansas
Lawrence
1962
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, Theodore H. Eaton, Jr., Henry S. Fitch
Volume 12, No. 5, pp. 241-296, 6 figs.
Published March 7, 1962
University of Kansas
Lawrence, Kansas
PRINTED BY
JEAN M. NEIBARGER, STATE PRINTER
TOPEKA, KANSAS
1962
29-1506
[Pg 243]
Natural History of the Bell Vireo,
Vireo bellii Audubon
BY
JON C. BARLOW
CONTENTS
| PAGE | |
| Contents | 243 |
| Introduction | 245 |
| Acknowledgments | 245 |
| Methods of Study | 246 |
| Study Area | 247 |
| Considerations of Habitat | 248 |
| Seasonal Movement | 250 |
| Arrival in Spring | 250 |
| Fall Departure | 251 |
| General Behavior | 252 |
| Flight | 252 |
| Foraging and Food Habits | 252 |
| Bathing | 253 |
| Vocalizations | 254 |
| Singing Postures | 255 |
| Flight Song | 255 |
| Daily Frequency of Song | 255 |
| Types of Vocalizations | 255 |
| Territoriality | 258 |
| Establishment of Territory | 259 |
| Size of Territories | 259 |
| Permanence of Territories | 260 |
| Maintenance of Territory | 260 |
| Aggressive Behavior of the Female | 264 |
| Interspecific Relationships | 264 |
| Discussion | 265 |
| Courtship Behavior | 267 |
| Displays and Postures | 268 |
| Discussion[Pg 244] | 270 |
| Selection of Nest-site and Nestbuilding | 272 |
| Building | 274 |
| Gathering of Nesting Materials | 276 |
| Length and Hours of Nestbuilding | 277 |
| Abortive Nestbuilding Efforts | 277 |
| Renesting | 277 |
| The Nest | 277 |
| Egglaying and Incubation | 278 |
| Egglaying | 278 |
| Clutch-size | 279 |
| Incubation | 280 |
| The Roles of the Sexes in Incubation | 280 |
| Relief of Partners in Incubation | 283 |
| Nestling Period | 283 |
| Hatching Sequence | 283 |
| Development of the Nestlings | 284 |
| Parental Behavior | 285 |
| Feeding of the Nestlings | 286 |
| Nest Sanitation | 287 |
| Fledging | 287 |
| Nest Parasites | 287 |
| Fledgling Life | 288 |
| Second Broods | 288 |
| Reproductive Success | 289 |
| Behavior | 290 |
| Predation | 291 |
| Cowbird Parasitism | 291 |
| Summary | 292 |
| Literature Cited | 294 |
[Pg 245]
INTRODUCTION
The Bell Vireo (Vireo bellii Aud.) is a summer resident in riparian
and second growth situations in the central United States south of
North Dakota. In the last two decades this bird has become fairly
common in western, and to a lesser extent in central, Indiana and
is apparently shifting its breeding range eastward in that state
(Mumford, 1952; Nolan, 1960). In northeastern Kansas the species
breeds commonly and occurs in most tracts of suitable habitat.
The literature contains several reports dealing exclusively with
the Bell Vireo, notably those of Bennett (1917), Nice (1929), Du
Bois (1940), Pitelka and Koestner (1942), Hensley (1950) and
Mumford (1952). Bent (1950) has summarized the information
available on the species through 1943. Nolan (1960) recently completed
an extensive report based on a small, banded population at
Bloomington, Monroe County, Indiana. He validated for this
species many points of natural history previously based on estimates
and approximations, especially concerning the post-fledging life of
the young and the movement of the adults from one "home range"
to another in the course of a single season.
None of these reports, however, has emphasized the ritualized
behavioral patterns associated with the maintenance of territory
and with courtship. Among the North American Vireonidae, the
behavior of the Red-eyed Vireo (Vireo olivaceus) is best documented
(Sutton, 1949; Lawrence, 1953; Southern, 1958). With this
species authors have concentrated on the mechanics of the breeding
season and their reports contain little discussion of the aggressive
and epigamic behavior of the bird.
The amount of information on the ritualized behavior of the Bell
Vireo and related species heretofore has been meager. I observed
breeding behavior from its inception in early May through the
summer of 1960. It is hoped the resulting information will serve
as a basis of comparison in future studies of behavior of vireos; such
ethological data are becoming increasingly important, especially as
an aid in systematics.
ACKNOWLEDGMENTS
To professors Frank B. Cross, Henry S. Fitch, and Richard F.
Johnston of the Department of Zoology of the University of Kansas
I am grateful for comments and suggestions in various phases of
the study and the preparation of the manuscript. Professor Johnston
[Pg 246]
also made available data on the breeding of the Bell Vireo from the
files of the Kansas Ornithological Society. I am indebted to my
wife, Judith Barlow, for many hours of typing and copy reading.
Mrs. Lorna Cordonnier prepared the map, Thomas H. Swearingen
drew the histograms, and Professor A. B. Leonard photographed
and developed the histograms. Dr. Robert M. Mengel contributed
the sketch of the Bell Vireo and George P. Young prepared the
dummy Bell Vireo used in the field work. Thomas R. Barlow,
Donald A. Distler, Abbot S. Gaunt, John L. Lenz, Gary L. Packard,
A. Wayne Wiens, and John Wellman assisted in various phases of
the field work.
METHODS OF STUDY
Daily observations were made from May 11 to June 26 in 1959
and from April 15 through July 15 in 1960. Six additional visits
were made to the study area in September of 1959, and ten others
in July and August, 1960. Periods of from one hour to eleven hours
were spent in the field each day, and a total of about five hundred
hours were logged in the field.
Each territory was visited for at least five minutes each day but
more often for twenty minutes. The breeding activities of the pairs
were rarely synchronous. Consequently several stages in the cycle
of building were simultaneously available for study.
Nine young and one adult were banded in 1959. No Bell Vireos
were banded in 1960. Individual pairs could be recognized because
of their exclusive use of certain segments of the study area
and by the individual variation in the song of the males. Sexes
were distinguishable on the basis of differences in vocalizations and
plumages.
Most nests were located by the observer searching, watching a
pair engaged in building, or following a singing male until the
increased tempo of his song indicated proximity to a nest. As the
season progressed and the foliage grew more dense, it became
increasingly difficult to locate completed nests. Blinds were unnecessary
because of the density of vegetation. Observations were
facilitated by a 7 × 50 binocular. Data were recorded on the spot
in a field notebook. Eggs were numbered by means of Higgins
Engrossing ink as they were laid.
Individual trees in which males sang most were marked over a
three-week period. Then the distances between the most remote
perches were paced. These distances aided in determining the
[Pg 247]
size of the territories. The general configuration of the vegetation
within each territory determined the location of one or more boundaries
of the territory. Each territory was given a number, 1, 2, 3,
etc., as it was discovered; consequently there is no numerical relationship
between the designations of the territories established in
1959 and 1960. Nests within a territory were designated as 1-a, 1-b,
1-c, etc.
Although experimentation was not a primary source of data, it
proved useful in certain instances. A stuffed Blue Jay elicited
mobbing behavior from nesting pairs. A dummy Bell Vireo elicited
both agonistic and epigamic behavior from nesting pairs, depending
on the phase of the nesting cycle.
The temperature at the beginning of each day's work was taken
by means of a Weston dial thermometer. A hand counter and a
pocket watch having a second hand were used in determining such
data as frequency of song and periods of attentiveness by the sexes.
Histological cross-sections, prepared by A. Wayne Wiens, of the
ventral epidermis of both sexes were used to study brood patches.
STUDY AREA
The intensive field work was on a 39-acre tract (fig. 1) extending
approximately 7/10 of a mile west from U. S. highway 59, which in
1959-1960 constituted the western city limit of Lawrence, Douglas
County, Kansas. The eastern boundary of the study area is
approximately 1-1/2 miles southwest of the County Courthouse in
Lawrence. The eastern ten acres is associated with the Laboratory of
Aquatic Biology of the University of Kansas. The 15 acres adjacent to
this on the southwest is owned by the University of Kansas Endowment
Association, but is used by Mr. E. H. Chamney for the grazing of
cattle. This portion is bounded on the west by a stone fence, beyond
which lies a 14-acre field of natural prairie owned by Dr. C. D. Clark
that is bordered on the extreme west by a narrow thicket of elm
saplings.
The principal topographic feature of the area is an arm of Mount
Oread, that rises some 80 feet above the surrounding countryside.
About 200 feet from the crest of the southwestern slope of the hill a
40-foot-wide diversion terrace directs run-off toward the two-acre
reservoir that is the source of water for eight experimental fish
ponds of the laboratory.
The predominant shrub-vegetation consists of Osage orange (Maclura
pomifera), honey locust (Gleditsia triacanthos), and
[Pg 248]
American elm (Ulmus americana). These saplings, ranging in height from
3 to 25 feet, grow in dense thickets as well as singly and in clumps of twos
and threes. Larger trees of these same species grow along the crest of
the hill, along the eastern and southeastern boundaries of the area,
and along the stone fence separating University land from that owned
by Dr. Clark. A dense growth of coralberry (Symphoricarpos
orbiculatus) forms the understory just below the crest of the hill.
Isolated clumps of dogwood (Cornus drummondi) and hawthorn
(Crataegus mollis) are scattered throughout the area. These species
of shrubs grow densely along the stone fence. The isolated thicket on
the Clark land is composed primarily of elm and boxelder (Acer
negundo), but includes scattered clumps of dogwood, Osage orange, and
honey locust. Poplars (Populus deltoides) are the only large trees
in this area.

Fig. 1. Map of the study
area near the University of Kansas Laboratory of Aquatic Biology. The
dashed lines mark the approximate territorial boundaries of the original
nine pairs of Bell Vireos from May 1960 to early June 1960.
The open areas between the thickets are grown up in red top (Triodia
flava), bluestem (Andropogon scoparius), Switchgrass (Panicum
virgatum), Kentucky bluegrass (Poa pratensis), bush clover
(Lespedeza capitata) and mullen (Verbascum thapsus). Shrubby
vegetation occupies about 65 per cent of the total area, but in the
Clark portion constitutes only about 35 per cent of the ground cover.
Considerations of Habitat
Nolan (1960:226), summarizing the available information on habitat
preferences of the Bell Vireo, indicates that this species tolerates
"a rather wide range of differences in cover." He pointed
[Pg 249]
out that a significant factor in habitat selection by this species may be
avoidance of the White-eyed Vireo (V. griseus) where the two species
are sympatric.
In Douglas County where the Bell Vireo is the common species, the
White-eyed Vireo reaches the western extent of its known breeding
range in Kansas. At the Natural History Reservation of the University
of Kansas, where both species breed, the Bell Vireo occurs in "brush
thickets in open places" (Fitch, 1958:270) and the White-eyed Vireo
occupies "brush thickets, scrubby woodland and woodland edge" (Fitch,
op. cit., 268). Along the Missouri River in extreme northeastern
Kansas, Linsdale (1928:588-589) found the White-eyed Vireo "at the
edge of the timber on the bluff, and in small clearings in the
timber," while "the Bell Vireo was characteristic of the growths of
willow thickets on newly formed sand bars." Elsewhere in northeastern
Kansas I have found the Bell Vireo in shrubbery of varying density and
often in habitat indistinguishable from that occupied by White-eyed
Vireos at the Natural History Reservation. In the periphery of the
region of sympatry the rarer species is confronted with a much higher
population density of the common species and consequently might well
be limited primarily to habitat less suitable for the common species.
This would seem to be the case in eastern Kansas, presuming that
interspecific competition exists.
The Bell Vireo has followed the prairie peninsula into Indiana, aided
by the development of land for agriculture. In nearby Kentucky where
thousands of miles of forest edge are found, and where little brushy
habitat of the type preferred by the Bell Vireo occurs, the White-eyed
Vireo is abundant whereas the Bell Vireo is unknown as a breeding bird
(R. M. Mengel, personal communication).
In more central portions of the area of sympatry, nevertheless, the
two species do occur within the same habitat (Ridgway, 1889:191; Bent,
1950:254) and occasionally within the same thicket (Ridgway, in
Pitelka and Koestner, 1942:105); their morphological and behavioral
differences, although slight, probably minimize interspecific
conflict. The Bell Vireo and the Black-capped Vireo (V.
atricapillus) have been found nesting in the same tree in Oklahoma by
Bunker (1910:72); the nest of the black-cap was situated centrally and
that of the Bell Vireo peripherally in the tree. Bell Vireos
invariably place their nests in the outer portions of trees and
peripherally in thickets. This placement would further obviate
interspecific conflict with the white-eye since its nests are placed
centrally in the denser portions of a thicket.
[Pg 250]
A critical feature of the habitat preferred by the Bell Vireo is the
presence of water. In far western Kansas this species is restricted to
riparian growth along the more permanent waterways. This in itself is
not adequate proof of the significance of water supply because thicket
growth in that part of the state is found only along waterways. The 20
areas over the state that I have visited where Bell Vireos were
present were closely associated with at least a semi-permanent source
of water. Fifteen other areas indistinguishable from the 20 just
mentioned, but lacking a permanent supply of water, also lacked Bell
Vireos. Nevertheless areas in which Bell Vireos typically nest are
decidedly less mesic than those frequented by White-eyed Vireos.
Once the Bell Vireo was probably more local in its distribution being
restricted to thickets associated with permanent water. Clearing of
woodland for agricultural and other use, and subsequent encroachment
of second growth concomitant with the creation of man-made lakes and
ponds, has greatly increased the available habitat for this bird. The
preferred species of shrubs for nesting are reported (Bent, 1950:254)
to be various wild plums (Prunus sp.). The widespread distribution
and abundance of the exotic Osage orange has greatly augmented the
supply of trees suitable for nesting.
SEASONAL MOVEMENT
Arrival in Spring
The subspecies of the Bell Vireo breeding in Kansas, V. b. bellii,
winters regularly from Guerrero and the Isthmus of Tehuantepec
south to Guatemala, El Salvador, and northern Nicaragua (A. O. U.
Check-list, Fifth Edition, 1957:469-470). In the United States
migrating birds are first recorded in early March (Cooke, 1909:119).
The Bell Vireo is a relatively slow migrator, moving primarily at
night and covering little more than 20 miles at a time (Cooke,
op. cit. 119). The average date of arrival, based on 27 records, for
northeastern Kansas is May 8; the earliest record is April 22, 1925,
from Manhattan, Riley County, Kansas (fig. 2-A).
In 1959 the first bird arrived at the study tract about May 5. No
additional birds were heard singing until the third week of the
month, in which eight new males were noted. As mentioned, in
1960 field work was begun in mid-April and the study area was
traversed daily. No birds were detected until late afternoon of
May 3, when one, presumably a male, was seen foraging.
[Pg 251]
Lawrence (1953:50) has reported that males of the Red-eyed
Vireo precede females in the breeding area by as much as two
weeks; the male Red-eyed Vireo forages but sings little in the pre-nesting
period. The male Bell Vireo arrives first at the breeding
area but precedes the female by only a few days. On the morning
of May 4 the first male was singing from a number of perches while
ranging over an area of seven acres. This area encompassed territories
later occupied by three pairs, 2 (1960), 4 (1960), and 5
(1960). Late on the afternoon of May 4 the first courtship songs
were heard and the first male was seen with a mate at 6:20 p.m.
Eight additional males arrived from May 6 through May 18. A
tenth male was discovered in the vicinity of territory 9 (1960) on
June 18, 1960.
Fig. 2. Seasonal movement as
indicated by the curve for spring arrival (A), based on the earliest dates for
27 years, and the curve for autumn departure (B), based on the latest dates for
21 years in northeastern Kansas.
Fall Departure
The average date of departure for 21 years in northeastern Kansas is
September 3 (fig. 2-B). The earliest date is August 14 from Concordia,
Cloud County, Kansas (Porter, unpublished field notes). The latest
date is September 27 (Bent, 1950:262) from Onaga, Pottawatomie County,
Kansas. In 1959 the last vireo was seen at the study tract on
September 14. The birds do not all depart at the
[Pg 252]
same time. On September 1 there were still five singing males in the
study area; by September 10 there were three and on September 13, only
one.
GENERAL BEHAVIOR
Flight
In "straight-away" flight the Bell Vireo undulates slightly. In a
typical flight several rapid, but shallow, wing beats precede a fixed-wing
glide of from 1 to 15 feet in length. Because the wings are
extended horizontally during the glide, the bird does not move distinctly
above or below the plane of flight. The White-eyed Vireo
generally appears to be slower and more lethargic in flight than the
Bell Vireo. In the breeding season most flights of the Bell Vireo
are no longer than a few feet between adjacent shrubs and trees,
but occasional sustained flights are as long as 300 feet. The birds
fly as low as 2 feet above ground, but have often been observed as
high as 70 feet above the ground.
In courtship and protracted territorial disputes, where chase between
sexual partners or a pair of antagonists occurs, looping flights
are observed. The wings are beaten as the birds climb and many
aerial maneuvers are performed in the course of the glide.
Foraging and Food Habits
The Bell Vireo has been characterized as a thicket forager (Hamilton,
1958:311; Pitelka and Koestner, 1942:104), but in my experience
it is not restricted to low level strata; birds forage from
ground level upward, both in thickets and isolated trees ranging in
height from 3 feet to 65 feet. The tendency to forage at higher
levels is in part dictated by the presence of tall trees within the
various territories.
Territories 1 through 7 (1960) contained from three to ten trees
surpassing 40 feet in height. They grew singly or in small groves.
The Bell Vireos foraged fully 20 per cent of the time in these trees.
Food was sought throughout the leaf canopy.
Behavior in foraging in larger trees followed a routine pattern.
Typically a pair alighted in a tree at a height of 15 feet. Then the
female hopped to a perch a foot above the one upon which she
landed. The male succeeded her to the perch she had previously
occupied. The pair in effect spiraled around some large, essentially
upright, branch, in foraging. The birds usually reached higher
[Pg 253]
perches in this manner rather than by flying upward 10 to 15 feet
to them. This manner of progression within a tree is reminiscent
of a similar habit of the Cyanocitta jays. Presumably, the habit of
the Bell Vireo of foraging in higher strata is facilitated by the absence
of other species of arboreal foraging vireos.
Chapin (1925:25) found the Bell Vireo to be more insectivorous
in its food habits than any other North American vireo. He found
99.3 per cent of all food contained in 52 stomachs to be of animal
origin. Only three times have I seen a Bell Vireo take food of
vegetable origin. On September 9, 10, and 14, 1959, I noted a male
eating wild cherries over a period of 65 minutes of observation.
Chapin (1925:27) noted that beginning in July vegetable matter
represented 1.57 per cent of the bird's subsistence, and thereafter
slightly more until fall migration.
Animal food, consisting primarily of insects and spiders, is actively
sought along branches and under leaves. Often a foraging bird will
leap to the underside of a branch and hover, mothlike, beneath a
cluster of leaves while extracting some insect. Some individuals
hung upside down on small branches, paridlike, while foraging.
Lawrence (1953:710), and Southern (1958:201) have recorded
similar behavior of the Red-eyed Vireo. Occasionally, I have seen
a Bell Vireo fly from a perch and capture an insect in the manner
of a flycatcher. The birds do not appear to be adept at this type
of food-getting. Nolan (1960:242) mentions Bell Vireos holding
hard-bodied insects by means of their feet while breaking the
exoskeleton with the beak to obtain the soft parts. Southern (1958:201)
recorded a female Red-eyed Vireo foraging on the ground; I
have seen a Bell Vireo on the ground but once, and it was gathering
nesting material.
Bathing
On May 14, 1960, in a rill that empties into the northeastern edge
of the reservoir a female flew down from a perch six inches above
the surface, barely dipped into the water, flew to a perch 12 inches
above the water, violently shook her ruffled body feathers, quivered
her wings, and rapidly flicked her fanned tail. The entire procedure
was repeated three times in five minutes. She was accompanied by
a singing male that did not bathe.
Nolan (1960:241) reports a male Bell Vireo bathing by rubbing
against leaves wet with dew; he notes that the White-eyed Vireo
bathes in a similar manner. Southern (1958:201) twice observed
[Pg 254]
Red-eyed Vireos bathing in water that dropped from wet leaves.
In my study area in 1960, only territories 7, 8, 9, and 10 were not
immediately adjacent to permanent water. The pairs of Bell Vireos
in those territories presumably had to reply on wet vegetation for
bathing.
VOCALIZATIONS
The male Bell Vireo begins to sing regularly soon after its arrival
in spring. Some daily singing continues following the cessation
of breeding activities until departure of the species in late
summer or early fall. The highest sustained rate of song occurs
on the first and second days of nest building. Because careful records
of meteorological data were not kept, I cannot significantly
correlate rates of song and specific temperatures and other weather
conditions. Frequency of song was reduced when the temperature
rose above 90° F., as it did on many days in June, 1960. Nice
(1929:17) mentions a similar decrease in singing when the temperature
exceeded 85° F.
Passerine birds typically sing at a high rate throughout courtship
and nestbuilding, but at a markedly lower rate thereafter. Most
vireos are atypical in this respect. In the study area in 1960 Bell
Vireos sang more often than Robins, Mockingbirds, Field Sparrows,
Brown Thrashers, Catbirds, and Doves breeding in the same habitat,
about as often as the Meadow Larks in the adjacent fields, and less
often than Painted Buntings.
The Bell Vireo seems to sing less often in the undisturbed state than
when aware of the presence of an observer. Observations from my car,
at a site approximately equidistant from territories 1 (1960), 2
(1960), 4 (1960), and 6 (1960) indicate that the rate of song during
incubation is decidedly less when no disturbing influence is present.
Normally, in this period, song aids in maintaining contact between the
members of a pair, serving to locate the male as he forages. Mumford
(1952:230) noted that the males often came out to meet him as he
entered their territories, singing as they approached. The male
typically continues to sing for some time after the intruder has
departed. Here the song acquires the additional functions of alerting
the female to danger and threatening the trespasser. Even after
allowance is made for this reaction to disturbance, Bell Vireos sing
more often than most of their nesting associates, and, on a seasonal
basis, they are vocal for a much longer time.
[Pg 255]
Singing Postures
In the normal singing posture the body of the Bell Vireo is maintained
at an angle of 35° to the horizontal. Occasionally, during
nest building, I have observed the body held at angles as severe as
80° from the horizontal.
The head of the White-eyed Vireo is distinctly bobbed up and
down, two or three times, during the utterance of a song phrase.
A bob involves a deliberate withdrawal of the head towards the
body and subsequent sharp, almost vertical, extension of the neck.
The head of the Bell Vireo does not bob, although it vibrates as the
song is delivered.
Flight Song
The Bell Vireo does not have a distinctive flight song; in fact, it
rarely sings or calls while in flight. Nolan (1960:240) has recorded a
male singing the normal song while in flight. Sharp scold-notes are
uttered in mid-air when a bird is agitated or actually attacking an
enemy. These notes and songs recorded by Nolan hardly qualify as
flight song, for this term implies use of a distinctive vocalization
not uttered in other circumstances.
Daily Frequency of Song
In the morning, Bell Vireos usually began singing a few minutes
before sunrise. Their songs were invariably preceded in the study
area by those of Western Kingbirds, Robins, Mourning Doves,
Mockingbirds, Cardinals and Meadow Larks. Bell Vireos sang relatively
little after 6:30 p.m., even on the longest days of the year.
The latest daytime singing that was recorded was seven songs at
7:18 p.m. on June 20, 1960. A Cardinal in the vicinity sang for
a full hour after this.
Types of Vocalizations
Six vocalizations were readily distinguishable in the field. These
are divisible into songs and call notes.
1. Primary song. It has been described by Pitelka and Koestner
(1942:103) as an "irregular series of harsh and sharp, but slurred
notes preceded by a few distinct notes of the same quality and
ending with a decided ascending or descending note of similar
harshness." The terminal note may also be somewhat abbreviated
and intermediate between an ascending or descending note. The
song is sometimes delivered as a couplet that consists of a phrase
ending on a descending note. This delivery is typical of incubation
and later renesting. During early season activities, the bird utters
[Pg 256]
a phrase ending on the descending note as many as 15 times before
a phrase ending on an ascending note is heard.
A sonagram of a single phrase, one of several recorded on May
9, 1960 (the third day of building of nest 1-b 1960), consists of
10 notes, the first of which is distinct. The remaining notes are
slurred. This phrase is 1.4 seconds in length.
Songs are delivered most rapidly in the course of territorial disputes
and defense. The song is loudest in times of nestbuilding and
periods of aggressive behavior. At these times, on clear, calm days,
the songs are audible 100 yards away. Singing in the nestling period
and post-breeding season is audible at distances of no more than
50 feet; such notes have been termed "whisper songs." Table 1 summarizes
singing rates at different periods of the nesting cycle in
several situations and under various weather conditions.
Songs are of equal frequency in the immediate vicinity of the
nest and elsewhere in the territory. Nice (1929:17) also found this
to be true. Perches can be almost at ground level or as high as 60
feet. Forty per cent of my data on song concern singing at heights
of more than 20 feet. As indicated in foraging, the lack of competition
from aboreal species of vireos presumably contributes to the use of
higher perches by Bell Vireos.
No female song was recorded in 1959, but on May 26, 1960, a
female was heard to sing once. She appeared at nest 1-f (1960)
shortly after the male arrived. Unlike him, she did not participate
in building, but seemed to be inspecting the nest. After 30 seconds
she sang once—a low garbled phrase—and also scolded once. After
this she left. In the meantime the continuously singing male moved
two feet away from the nest, then back to it and resumed construction.
The song of the female signaled to the male her departure.
Pitelka and Koestner (1942:103) heard a female sing twice after
she replaced the male on the nest. Females of three other species
of vireos, the Black-capped Vireo, V. atricapillus (Lloyd, 1887:295),
the Philadelphia Vireo, V. philadelphicus (Lewis, 1921:33), and the
Latimer Vireo, V. latimeri (Spaulding in Pitelka and Koestner,
1942:103) have been heard singing. Lewis and Spaulding also suggest
that the song of the female functions as a signal prior to
exchange at the nest.
The primary song identifies the singer as a male Bell Vireo. It
aids in securing a mate and in warning potential adversaries; also,
the song is a signal in certain situations and serves to locate the male.[Pg 257]
Table 1. Representative Singing Rates of Breeding Bell Vireos. All
Rates Were at Air Temperatures Less Than 86° F. Each Instance Represents
Approximately 30 Minutes of Observation.
| Circumstance | Instances | Average rate per minute |
|---|---|---|
| Attraction of mate | 2 | 6.3 |
| Territorial dispute | 5 | 12.8 |
| Nestbuilding | 6 | 7.0 |
| Egglaying | 1 | 3.0 |
| Incubation | 6 | 3.9 |
| Exchange of partners in the incubation period | 1 | 4.0[A] |
| Foraging | 2 | 2.2 |
| "Morning" song | 1 | 28.6[A] |
| "Evening" song | 1 | 1.9[A] |
| Overall average rate per minute 6.3 | ||
[A]
Not sustained; data representative of periods less than 5 minutes in length.
2. Courtship song. It is here termed the "congested" song and
is comparable to the adult "run-on" song mentioned by Nolan (1960:240).
The congested song is a squeaky version of the primary song
and is given when birds are engaged in pair-formation, nestbuilding,
and egglaying. The delivery is rapid and the sound can be likened
to that made by rapidly scraping a bow across a taut violin string.
Nolan (in Mumford, 1952:230) is probably speaking of this song
when he describes a "tuneless" song that "had a jerky, sputtering
quality that characterizes part of the song of the Ruby-crowned
Kinglet (Regulus calendula)." More recently (1960:240) he applies
the adjectives "twanging," "Bobolink-like," "bubbling," "jerky," and
"squeaky." This song is often blended with the primary song and
is audible for 75 feet.
A specialized version of the congested song is associated with
pre- and post-copulatory display but differs from the typical squeaky
performance in terminating in two ascending notes reminiscent of the
ascending phrase of the primary song.
3. Distress call. It was heard only once, when a captured bird
was being freed from a net. When the bird was almost disentangled
it uttered 10 high-pitched, plaintive notes. The quality of the notes
suggested a relationship to the song phrase rather than to other
types of vocalization. A nesting pair of Bell Vireos, 10 feet away,
became extremely excited when they heard the distress notes. They
"scolded" vigorously and flew around my head at a distance of
six feet.[Pg 258]
4. Alarm note. This is a specialized, three-note call of the male
and was heard only from the onset of pair-formation through
early nestbuilding. This whinnying, flickerlike call, phonetically
eh-eH-EH, each succeeding note of which is louder than the one
before, is given whenever the male is disturbed by an unfamiliar
object. This call is generally succeeded by the chee, but occasionally
blends into an extended "whinny," and is typically given from
some perch affording an unobstructed view of the offending object.
The male stretches his neck and cocks his head, the wings and tail
are not flicked or fanned, and no feather tracts are erected. The
bird, nevertheless, flits nervously from perch to perch when uttering
the call.
5. The zip. The male has a special "scold" note of his own that
is heard when an intruder first approaches the nest. Phonetically
it is zip-zip-zip. It is not so loud as the chee, and the delivery is
more deliberate than that note. If the intruder remains near the
nest, the zip is usually replaced by the chee.
6. The generalized call note or chee. The call notes associated with
several situations are combined under this subheading since all can be
rendered in English by the same phonetic equivalent—chee. The
chee associated with nestbuilding is of moderate pitch and delivered
deliberately at a rate of about 40 per minute. The feeding call of the
adults is a soft slurred chee, while that of the nestlings has a
mewing quality. In general, the chee utilized in signal situations
consists of a few repetitions of the basic note emitted at a moderate
pitch. The chee associated with hostile and courtship behavior is
higher pitched and the delivery is much more rapid, approximately 200
per minute. Nolan (1960:240) reports a continuous rate of 25 per five
seconds when an adult Bell Vireo is alarmed. The chee of extreme
anxiety is a loud emphatic buzz, phonetically ZZ-ZZ-ZZ-ZZ.
TERRITORIALITY
The Bell Vireo exhibits "classic" passerine territoriality. Within
a specific area, a pair of this species carries out pair-formation,
courtship activities, copulation, nesting, rearing the young, and
foraging. With the cessation of reproductive activities, a pair continues
to restrict its other daily activities to the same general area.
[Pg 259]
Establishment of Territory
In early May the segment of the total suitable habitat within
which a Bell Vireo restricts its activities is not rigidly defined and
the first male of the season ranges over an area too large to be
maintained permanently—one that seems greatly to exceed the
needs of breeding. Male 1 (1960), for instance, was first seen foraging
over an area of approximately seven acres. With the influx
of other males, portions of this large tract were usurped and the
territory of the original male was gradually reduced to an area
of little more than an acre.
In this initial period, a male becomes identified with a large area
but is restricted to an area of nearly typical size by the
encroachment of other males. Territorial disputes in this period often
involve physical contact, as well as protracted sessions of
high-intensity singing at rates exceeding three hundred song-phrases
per hour.
Eventually the carrying capacity of the habitat is reached and
no further partitioning occurs. The beginning of nestbuilding
coincides with this relative stabilization of the territorial boundaries.
Through the remainder of the cycle of behavior associated with any
one nest, all activity is that of the occupant pair within its territory.
Size of Territories
The nine original territories established in 1960 varied in size from
0.26 acre to 3.1 acres (Table 2). Fitch (1958:270) found the
territories of several pairs of Bell Vireos at the University of
Kansas Natural History Reservation to vary from 0.4 to 1 acre. Hensley
(1950:243) estimated the size of the territory of a pair of Bell
Vireos observed in Piatt County, Illinois, at 3.1 acres. Nolan
(1960:227) records home ranges of 2 to 3 acres. The pairs that he
studied were sole occupants of fields several acres larger than the
portions actually utilized. His description of the vegetation
indicates that most of the second growth was not much taller than 7
feet. As indicated elsewhere, the second-growth in my tract averaged
15 feet tall. The smaller average size of territory (1.25 acres) that
I found is probably a function both of this greater vertical range of
available foraging area and the much higher gross density of birds (40
pairs per 100 acres).
[Pg 260]
Permanence of Territories
Most pairs remain in their original territories throughout the
summer, although some shift certain territorial boundaries. In
1960 pairs 2 and 6, in the course of selecting a site for a replacement
nest, annexed adjacent areas previously occupied by other
pairs. Pair 2 relocated in a space that originally included territories
1 and 4, and pair 6 built a nest in an area formerly occupied by
pair 7. Males 1 and 4 were sacrificed for specimens and pair 7
probably was destroyed by a predator. Owing to the presence of
a nest, the annexed area becomes the focal point of the activities
of a pair, but the original area is regularly visited and may be returned
to in a later renesting.
Table 2. Size of the Nine Original Territories Occupied in 1960.
| Territory | Date first occupied | Dimensions |
|---|---|---|
| 1. | May 3, 1960 | 1.6 acres |
| 2. | May 5, 1960 | 0.6 acre |
| 3. | May 7, 1960 | 0.26 acre |
| 4. | May 11, 1960 | 1.03 acres |
| 5. | May 12, 1960 | 2.07 acres |
| 6. | May 14, 1960 | 3.1 acres |
| 7. | May 13, 1960 | 1.7 acres |
| 8. | May 14, 1960 | 0.46 acre |
| 9. | May 14, 1960 | 0.4 acre |
| Average 1.25 acres | ||
Maintenance of Territory
Except in the early stages of nesting, territory is maintained
primarily by song. In the period of incubation a male regularly
patrols his territory between sessions of sitting on the eggs. He
sings several songs from each of several perches. A male follows
a predictable path, rarely traveling more than 150 feet from the
nest. Incipient patrolling is seen early in the breeding season
when territorial boundaries are in a state of flux.
The male White-eyed Vireo travels a semi-predictable route, as does
the Solitary Vireo (R. F. Johnston, MS). According to Lawrence
(1953:50), the male Red-eyed Vireo has a distinct singing area
completely divorced from the nest area dominated by the female.
Southern (1958:109), working with this same species in Michigan, did
not recognize separate areas, but found that the male wandered
randomly over the territory.
[Pg 261]
In a species so highly active as the Bell Vireo, the degrees of
hostile action associated with an encounter overlap in such a
fashion that no clearcut distinction can be drawn among the various
displays. Nevertheless, certain generalized patterns are characteristic
of all situations in which members of this species are in a state
of anxiety. The threat displays described in the succeeding paragraphs
may all be utilized within as little as two minutes; mutual
agonism may be terminated at any stage by concerted attack of the
dominant bird.
1. Vocal threat. When an intruder is discovered the resident
male markedly increases his rate of singing. The alarm note, eh-eH-EH,
is the first call uttered during the nestbuilding and egglaying
periods.
2. Head-forward threat. If the intruder does not flee, the resident
male adopts a specific threat posture. The head and neck
are extended. The feathers of the crown are erected, but those of
the body are sleeked. The bird crouches slightly and the tail is
flicked laterally, but not fanned. The intensity of the singing increases
and is supplemented by scolding, also delivered at a rapid
rate. The intruder normally retreats at this juncture.
3. Wing-flicking and submaximal tail-fanning. If the interloper
remains, the anxiety of the resident male increases. He slightly
depresses the tail and, at the same time, rapidly fans and closes it.
The tail is only partially fanned. The wings are held slightly away
from the body and rapidly flicked above the back. This flicking
should not be confused with quivering of the wings associated with
begging and other solicitory postures. Song is now almost completely
replaced by high-intensity scolding. Associated with this
high degree of anxiety are displacement behaviorisms, including
bill-wiping, reversal of direction on a single perch, and a nervous
hopping from one perch to another.
4. Ruffling and maximum tail-fanning. This display is most
often seen in conjunction with the harassment of predators, but
occasionally it is observed in territorial disputes occurring at the
boundary of adjacent territories where neither male is strictly
dominant and in which there is much vacillation prior to attack.
The feathers of the abdomen are ruffled. The term "ruffled" pertains
to a full erection of the feathers, giving a ragged appearance
to the body outline (Morris, 1956:80). Ruffling of the abdominal
feathers emphasizes their yellow color and seemingly heightens
the intimidatory effect. The tail is fully fanned, and so maintained,
[Pg 262]
for a few seconds at a time; it is held at a 45° angle to the body.
The scold becomes an extremely intense, stacatto buzz, ZZ-ZZ-ZZ-ZZ.
5. Supplanting attack. The attack directed against a trespassing
male is initiated as a lunge that results in a collision with the opponent
in mid-air or on his perch. The bird attacked is struck by
his adversary's open beak or body.
Hinde (1952:71-72) indicates four courses of action followed by a
Great Tit (Parus major) when attacked under similar circumstances.
"(a) It flies away: The attacker usually flies after it and
a chase ensues. (b) It shifts its perch a few inches: the attacker
lands in its place, and both usually show head-up postures. (c)
It remains where it is, but adopts a head-up posture: the attacker
usually then shows upright flight. (d) It may fly up and meet
the attacker in mid-air: in that case an actual combat may result,
or both combatants may show upright flight."
Head-up posturing and upright flight are not presently recognized
components of the behavior of the Bell Vireo. The behavior of the
attacked Bell Vireo is similar to that described in (a), (b), and
(d) above, and is clearly dictated by the proximity of his own
"home base."
Eleven disputes among occupants of adjacent territories were
witnessed between May 6 and June 3, 1960, in which some or all
of the described threat displays were manifest (Table 3). In each
instance, patrolling males were gradually attracted to each other.
As they approached, their rates of song increased from an average of
six repetitions per minute to 15 per minute. Eight of the disputes
involved physical combat.
On May 6, 1960, when male 2 (1960) was in the process of usurping
an eastern segment of the original territory of male 1 (1960),
a violent, protracted dispute was observed. By this date male 1
(1960) had obtained a mate and had begun construction of nest
1-a (1960); male 2 (1960) had not yet acquired a mate. At first
the two males were singing vigorously, from one to 10 feet apart.
Female 1 (1960) followed her mate closely and scolded, at the same
time partially fanning her tail. In the course of vocal dueling the
males had traveled to within 50 feet of nest 1-a (1960), when male 1
(1960) suddenly lunged at 2 (1960). The males plunged to the
ground, locking bills and clutching at each other with their feet as
they fell. As soon as they touched the ground they separated.
[Pg 263]
Male 2 flew east with male 1 in pursuit. This conflict lasted three
minutes.
Additional physical combat was witnessed several minutes later.
This again involved striking with the bill, wings and feet. A high
pitched squeaky chee was uttered by both combatants. The female
scolded from a nearby perch. Upon separating, the males engaged
in a wild, looping flight. At about 350 feet from nest 1-a (1960),
the chase abruptly ended. For ten minutes thereafter, both males
sang at a high rate from perches about 10 feet apart. This terminated
the physical combat, but three additional protracted, vocal
duels occurred in the remainder of the morning.
Table 3. Intraspecific Disputes in Maintenance of Territory.
Behavior
| Number of conflicts | Vocal dueling | Combat | Average length of disputes | |
|---|---|---|---|---|
| Prenesting | 3 | 3 | 2 | 6 min. 40 sec. |
| Building | 8 | 8 | 6 | 3 min. 8 sec. |
| Incubation | 1[B] | 1 | ... | 20 min. |
| Totals | 12 | 12 | 8 | 5 min. 30 sec. |
[B]
Directed against a stuffed Bell Vireo.
Probably as a direct result of these conflicts, a neutral zone
approximately 300 feet wide developed between the two territories. By
May 14 this intervening area was occupied by male 4 (1960). By this
date both 1 (1960) and 2 (1960) were involved in nestbuilding and 4
(1960) was not challenged for several days.
Maximum tail-fanning prior to attack also appears as an element
of aggressive behavior in White-eyed Vireos. A brief skirmish between
a male of this species and a small, greenish passerine was
observed at the Natural History Reservation on May 25, 1960. The
White-eyed Vireo was singing from a perch 30 feet high in a dead
elm, when the unidentified passerine landed 10 feet distant. The
white-eye ceased regular song and uttered several catbirdlike calls,
and at the same time slightly depressed and fully fanned the tail.
After 10 seconds, the white-eye lunged at the intruder, striking it in
mid-air. A brief looping flight ensued through the branches of the
elm before the intruder was able effectively to retreat.
[Pg 264]
Aggressive Behavior of the Female
The female Bell Vireo is concerned primarily with the defense of
the nest and the young and she rarely assists the male in defense
of distant parts of the territory. She employs the same threat displays
as the male.
Interspecific Relationships
A number of meetings between Bell Vireos and other species were
observed in the course of the study (Table 4). Resident pairs of
this species exhibited different degrees of tolerance toward other
species. Many birds, including Cardinals, Field Sparrows, Painted
Buntings and Mourning Doves were ignored completely. Chickadees
evoked responses characterized by slight increase in song and
some anxiety; this was perhaps owing to similarity in size, motion
and call notes. Warblers, when met with, were invariably chased.
They may be momentarily mistaken for rival vireos.
Table 4. Interspecific Conflict Observed in 1959 and 1960.
| Species | Number of conflicts | Phase of breeding cycle | Behavior of Bell Vireos | |||
|---|---|---|---|---|---|---|
| HFT[C] | S | TF | A | |||
| Coccyzus americanus | 1 | Nestling period | x | |||
| Cyanocitta cristata | 3[D] | Nestling and incubation period | x | x | x | x |
| Parus atricapillus | 1 | Prenesting | x | |||
| Molothrus ater | 1 | Nestling period | x | x | ||
| Dendroica petechia | 1 | Prenesting | x | x | ||
| Geothlypis trichas | 1 | Nestbuilding | x | x | ||
| Pituophis catenifer[E] | 1 | Post-fledging | x | x | ||
[C]
HFT = head-forward threat; S = scolding; TF = tail-fanning; A = attack.
[D]
Includes attack against a dummy Blue Jay.
[E]
The Bull Snake is here included because the vireos directed typical aggressive displays towards it.
Blue Jays were vigorously attacked, especially late in incubation
and throughout the nestling period of the Bell Vireo. I did not see
a jay struck, but a vireo would circle one closely as it perched and
pursue it when it flew, following as far as 100 yards beyond territorial
bounds. The buzz, ZZ-ZZ-ZZ-ZZ, was uttered in conjunction
with this harassment.
A stuffed jay placed eight feet from a nest elicited threat display
and displacement behavior from the owners of the nest, but no
[Pg 265]
attack. Incubation had just begun at this nest. A dummy Bell Vireo
placed close to another nest only momentarily disturbed the male,
and the female completely ignored it. Incubation had also recently
begun at this nest. At this same general stage, moreover, nesting
pairs showed little inclination to harass me.
Discussion
Hinde (1956:341-342) indicates that territory has been defined
in a number of ways by many workers. All of the definitions involve
modification of Howard's classic "defended area." Pitelka (1959:253)
has reacted against this behaviorally-oriented concept. He
thinks that the concept of territory should be based on exclusive
use of an area by its occupants, and not so much the defense by
which they maintain it.
Methods of treating territoriality in the Bell Vireo seemingly
incorporate features of both schools of thought. The area used
exclusively for all biological needs by a single pair of Bell Vireos
is vigorously defended both physically and vocally early in the
breeding season and vocally as the season progresses.
In the period of territorial establishment a relatively large area
is actively defended. The building of a nest establishes a focal point
of activity in a somewhat more restricted area than that originally
occupied. After the success or failure of a nest, a new site is selected
to which the focal point of activity is shifted. If suitable habitat
adjacent to the extant territory is unoccupied by other Bell Vireos
the unoccupied area may be annexed in the course of searching for
a new site. Such annexation occurs only when pairs formerly occupying
adjacent suitable habitat disappear from this territory;
possibly the size of the territory of any one pair is dictated by the
density of population of the species as well as by the presence of
suitable habitat. This may not always be true as indicated by
Kliujver (1951:40), who in studying the Great Tit, found no appreciable
difference in the size of territory in two different habitats
even though there was a marked difference in population density
of the birds.
Fluctuation of territorial boundaries is not uncommon in passerines,
especially when no rivals exist to contest movement. Hinde
(1956:351) indicates that fluctuations in size of territory are to be
expected although the territories of different species of birds have
different mean sizes.
Once nesting activities commence there is a marked reduction in
[Pg 266]
the amount of territory utilized and a distinct decrease in the
aggressive tendencies of the male; it would seem that energy previously
utilized in regular fighting is rechanneled for nestbuilding,
incubation and care of the young. Further, contraction of the area
of activity obviates high-intensity territorial defense, as adjacent
males, even in regions of high population density, are isolated from
one another by an area no longer regularly traversed.
With cessation of breeding activities physiological mechanisms
governing maintenance of territory seemingly are no longer active
and yet the pairs of Bell Vireos remain within a restricted area which
they alone use. Earlier definitions of territory as a "defended area"
do not adequately cover such situations and yet from the standpoint
of Pitelka the area still retains the characteristics of true territory.
In fact, territory as defined by Pitelka is clearly manifest at this
time. Whether the birds remain in an area through "force of habit"
is of little consequence.
I have retained the term "territory" in preference to the term
"home range" used by Nolan (1960:227). His failure to observe
territorial defense is responsible for his terminology, although it is
readily understandable that such defense would be lacking in a
population of relatively low density in which pairs were isolated
from one another by areas of unfavorable habitat. This isolation in
itself would tend to preclude territorial conflict but territories were,
in fact, maintained.
The marked similarity in the essential features of aggressive
behavior in North American vireos attests to their close relationship.
Flicking and fanning of the tail are distinct components of the hostile
behavior of the Bell Vireo, White-eyed Vireo, Red-eyed Vireo (Lawrence,
1953:69), and the Black-whiskered Vireo (Vireo altiloquus;
Bent, 1950:319), and, presumably, of the remaining species of the
genus. The occurrence of these same displays as intrinsic behavioral
elements of interspecific hostility suggests a common derivation.
Moynihan (1955:256) indicates that all intraspecific hostile displays,
and probably most interspecific hostile displays, evolved originally
as social signals having the same general function. Further, Hinde
(1956:344) points out that there is a fundamental similarity in the
motor patterns used in fighting in different contexts, including both
interspecific and intraspecific fighting.
[Pg 267]
COURTSHIP BEHAVIOR
The precise mechanism of pair-formation in the Bell Vireo is not
known. My experience has been to find a male one day and then
one or two days later to discover that it has a mate. Lawrence
(1953:53), tells of a male Red-eyed Vireo singling out a female
from a flock of migrants passing through his territory and violently
driving her to the ground. Shortly after this attack the pair was
seen searching for a nest site. But such an incident has not been
reported for other vireos, nor have I witnessed such behavior myself.
Early courtship activities of the Bell Vireo are characteristically
violent affairs, with the male directing strong aggressive attacks
toward the female. Rapid, looping flights through the thickets
occur, the female leading the male. Occasionally he deliberately
collides with her in mid-air, but the pair quickly separate. This
violent sexual chasing is manifest prior to the inception of nestbuilding.
With commencement of this activity, sexual chases through
the territory subside.
Absence of sexual dimorphism in the Bell Vireo obviously suggests that
behavioral criteria are used by the birds in sex-recognition. The lack
of aggression by the female upon initial aggression by the male is an
essential component of recognition of sex; she is clearly subordinate.
Such subordination is also the significant feature of continued
sex-recognition. Courtship display by a resident male, directed toward
a stuffed male and a wounded male which sat motionless, supports the
contention that a subordinate or submissive attitude of the female is
a key factor in sex-determination.
Nestbuilding and courtship are intimately associated in this
species. The male constructs the suspension apparatus of the nest,
the completion of which coincides with the assumption of nestbuilding
activity by the female. Roles of the sexes in nestbuilding are
described in the section on nestbuilding. The male frequently interrupts
construction to court the female. This, in combination with
perpetual song as he works, serves to strengthen the pair-bond and
stimulate nestbuilding tendencies of the female.
It is doubtful that any attempts at copulation are successful up
to this time. The female is singularly unresponsive to the advances
of the male; a female retreats before most violent attacks and is
seemingly oblivious to less vigorous behavior. After the female
[Pg 268]
assumes the responsibility of building, the tempo of courtship
activities increases.
The female becomes increasingly more receptive and her work is
often interrupted by advances of the male. Copulation occurs frequently
from about the third day of nestbuilding through the first
day of egglaying, a period of four to six days. Male displays and
vocalizations associated with courtship continue through the fourth
or fifth day of incubation.
Displays and Postures
The principal courtship displays and postures that were seen
throughout the nestbuilding phase are as follows:
1. Greeting ceremonies. Both birds are crouched from one to five
inches apart. The feathers on one (the male?) are sleeked, and on
the other are fluffed. Fluffing (Morris, 1956:80) denotes partial
erection of the body feathers producing a rounded, unbroken body
line and is not to be confused with ruffling, mentioned in the sections
pertaining to territoriality and pre- and post-copulatory display.
Fluffing is generally considered to be an appeasement display and
it is seen in a variety of situations involving a dominant-subordinate
relationship. Both birds flick wings and tails rapidly and reverse
directions on their perches frequently. A low, rapid chee is uttered
during this performance. This ceremony is repeated often in the
first three days of nestbuilding, but less frequently thereafter. It
usually occurs after building by one or both partners and prior to
another trip in search of nesting material. It lasts from 10 to 50
seconds and is not immediately followed by any additional courtship
activities. Nolan (1960:228-229) observed mutual displays between
periods of violent sexual chase that suggest that the greeting ceremonies
that I have described are an integral part of pair-formation
as well as a component of continued maintenance of the bond.
2. "Pouncing." The female rapidly quarter-fans and partially
depresses her tail. She utters a high pitched scold (chee). The
male, from a perch within two feet of the female, fans the tail fully
and depresses it vertically, and, with mouth open, lunges at the
female; or, with similar tail mannerisms, the abdominal feathers
ruffled, the wings held horizontally, and the primaries spread, he
sways from side to side from four to six times, and then lunges at
the female. The male is silent when he pounces; the chee or the
courtship song is emitted when swaying precedes pouncing. The
male strikes the female with his breast or with his open beak. The
female rarely flees although she is usually displaced several inches
[Pg 269]
along the branch upon which she is sitting. However, the female
may fly several inches to a new perch. The failure of the female to
adopt a solicitation posture presumably indicates sexual unreadiness.
Instances of the male deliberately colliding with the female
as she flies in the course of gathering nesting material are probably
analogous to pouncing. In none of the above situations are females
observed to fight back in any way. Nice (1943:174) believed pouncing
to be analogous to sexual chasing found in such species as the
Red-winged Blackbird. In the Song Sparrow, pouncing is observed
most often in the first and second days of nestbuilding.
3. "Leap-flutter." The male, in the course of displaying with the
tail fanned before the female, suddenly leaps eight inches to ten
inches vertically and flutters in mid-air several seconds, before dropping
to the original perch. This display occurs in full view of the
female. It is often associated with pouncing and is also seen prior
to copulation. In the latter instance it is probably pragmatically
functional, for it permits the male to orient above the female before
dropping to her back to copulate. No vocalization is uttered during
the leap-flutter.
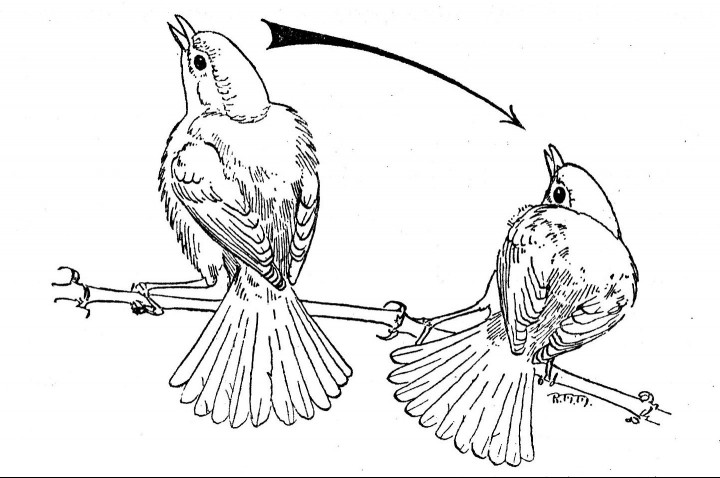
Fig. 3. A single male Bell Vireo
in the pre-copulatory display. Note the ruffled dorsal and ventral body feathers.
The male on the left has reached the zenith of a single swing. The male on the
right has nearly reached the low point of a swing.
4. Pre-copulatory display (Fig. 3). The male faces the female.
The tail is fanned fully and depressed at a sharp vertical angle to
the body. Body feathers, both dorsal and ventral, are ruffled, almost
tripling the apparent volume of the thorax. The head is withdrawn
and slightly thrown back. Feathers of the head are not erected.
[Pg 270]
The mouth is opened wide. The legs are slightly flexed and the
body is swayed laterally. Horizontally, the head and body traverse
an arc of about 100°; vertically, they traverse an arc slightly less
than 180°. At the low point of any one swing, the delivery of the
courtship song begins. At the termination of the swing the two
normal, ascending notes are emitted. This performance may last
as long as three minutes.
The pre-copulatory display of the male elicits receptive behavior
in the female. She crouches in a solicitous manner, with the body
feathers fluffed and the tail raised slightly, and utters a muted chee.
5. Copulation. The male abruptly terminates his swaying display
with a leap-flutter that positions him above the female's back. He
then descends and copulation occurs. The male continues to flutter
his wings to maintain balance throughout the two seconds of cloacal
contact. Following an unsuccessful copulation on June 23, 1960,
displacement preening and bill wiping were performed by both
sexes.
6. Post-copulatory display. On June 25, 1960, after a second
attempt at copulation with a stuffed bird in which semen was
actually deposited on the dummy's back, male 10 (1960) performed
a swaying display. In this instance, however, instead of addressing
the dummy from the front, the male alighted one inch to the right
of the stuffed bird. When swaying to the left (toward the dummy)
the head of the displaying male actually passed above the neck of
the stuffed bird. This ritualized behavior could conceivably be
derived from hetero-preening.
Discussion
Within the scope of my research it was difficult to detect the
over-all sequence of epigamic displays that result in synchronization
of the physiological states of the sexes throughout the period of
courtship. Possibly all displays, except the post-copulatory one,
occur in no particular order in the courtship period. However, each
ritualized display seemingly strengthens the pair-bond.
Swaying has been recorded in a variety of situations of a sexual
and semi-sexual nature for the Solitary Vireo (V. solitarius; Townsend,
1920:158) and the Red-eyed Vireo (Tyler, 1912:230; Bent,
1950:342). In every instance the body feathers of the swaying
birds were sleeked. Courtship behavior in any species of North
American vireo seems closely to resemble that of any other; pairing
[Pg 271]
and nestbuilding of a female V. solitarius and a male V. flavifrons
as reported by Hauser (1959:383) support the idea of close resemblance.
A marked similarity will be detected between certain basic elements
of aggressive and epigamic displays. These basic elements
are wing- and tail-flicking, tail-fanning, and high-intensity delivery
of the chee. Pouncing and supplanting attacks are essentially similar.
Such similarities suggest either a common origin for certain
aggressive and epigamic displays or the derivation of one from the
other.
High-intensity cheeing is obviously a function of excitement,
whether in conjunction with hostility or sexual behavior. According to
Andrew (1956:179), flicking of wing and tail in passerines are
intention movements of flight. These actions have been emancipated
from incomplete take-offs and incorporated in ritualized courtship and
agonistic behavior. In incipient courtship behavior the male is
governed by three conflicting tendencies; to flee, to attack, or to
behave sexually before his mate (Tinbergen and Hinde, 1958:256). When
pairing, Bell Vireos interrupt sexual chase with "greeting
ceremonies," the male's tendency to attack and the female's tendency
to flee are momentarily reduced, and the forming bond is strengthened.
Thus, the intention movements become an integral part of courtship.
In situations where attacking and fleeing are the two conflicting
tendencies, wing-flicking and tail-flicking are incorporated into
threat display, but do not lose all of their original function, for
they facilitate attack. Tail-fanning, as a display element, increases
the awesome aspect of the threatening bird and in courtship presumably
makes the sexes more attractive to one another.
Courtship feeding has not been recorded for the Bell Vireo. In
general, it is unknown in North American vireos, with the exception
of the red-eye (Lawrence, 1953:53). It would serve no "practical"
purpose in the Bell Vireo since the male regularly relieves the
female during incubation, thus allowing her ample opportunity to
forage. In the Red-eyed Vireo, only the female regularly incubates,
and courtship feeding is definitely functional. Nolan (1960:228)
described a brief pecking or pulling with their bills between
pairing birds. This may be incipient "symbolic" courtship feeding,
or perhaps mutual preening.
[Pg 272]
SELECTION OF NEST-SITE AND NESTBUILDING
As far as can be determined, the nest-site is selected by the
female. Typically, the pair makes short, low-level flights from tree
to tree with the female invariably in the lead. The birds usually
forage within each tree; the female interrupts this activity to inspect
small forks of low, pendant branches and the male occasionally
pauses to sing. The singing is loud but not particularly regular,
as it is later when the male accompanies the female during actual
nestbuilding. Method of selection of site resembles that described
by Lawrence (1953:53) for the Red-eyed Vireo.
Nests are suspended from lateral or terminal forks about 27 inches
high in bushes and small trees that, in the study area, averaged
11 feet, four inches in height (Table 5). The height above ground
of the nests does not vary appreciably as the season progresses as
is the case with nests of Red-eyed Vireos, for which Lawrence
(1953:54) noted that late nests were placed higher than those
built earlier in the season.
Most nests are so situated that they are protected and concealed
by the dense foliage of trees. Where nests are placed in low bushes,
as coralberry or dogwood, the bush is invariably overhung by the
foliage of a much taller shrub or tree.
The nest tree or shrub was in every instance situated at the edge
of a thicket or isolated from adjacent trees by several feet. Preference
for open situations is characteristic of the species. In contrast,
the nest of the White-eyed Vireo (Bent, 1950:229) is placed
toward the center of thickets.
In the choice of sites in the study area, the Bell Vireos were
almost unopposed by other avian species, owing to the size of the
[Pg 273]
fork utilized and the fact that the nests are located peripherally,
rather than centrally, in the bush or tree. This lack of competition
for a nest-site provides a Bell Vireo with an ample supply of nest-sites
within any one territory.
Table 5. Nest-sites Utilized in 1960.
| Plant | Number of nests | Average height of plant | Average height of nest |
|---|---|---|---|
| Ulmus americana | 4 | 7 ft. 6 in. | 2 ft. 3 in. |
| Maclura pomifera | 20 | 13 ft. 11 in. | 1 ft. 11 in. |
| Crataegus mollis | 1 | 11 ft. | 3 ft. 1 in. |
| Gleditsia triacanthos | 2 | 15 ft. 6 in. | 1 ft. 9 in. |
| Acer negundo | 4 | 8 ft. 9 in. | 2 ft. 5 in. |
| Cornus drummondi | 2 | 8 ft. | 2 ft. 8 in. |
| Symphoricarpos orbiculatus | 3 | 3 ft. | 1 ft. 10 in. |
| 7 | 36 | 11 ft. 4 in. | 2 ft. 3 in. |
Selection of the first nest-site may take as long as two days,
possibly owing to incomplete development of the nesting tendency,
but more likely to a general lack of familiarity with the territory.
Red-eyed Vireos require five to six days to choose the first nest-site
(Lawrence, 1953:54). Later sites of the Bell Vireo are chosen in
as little as three hours. Nest 1-c (1960) was abandoned at about
11:00 a.m. on May 14, 1960, when part of the thicket on the edge
of which this nest was located was removed by brush-cutters clearing
a power line right-of-way. By 2:00 p.m. this pair had begun
construction of 1-d (1960) in an Osage orange 110 feet southwest of
1-c (1960).
This particular site is of further interest because it is the same
one utilized for nest 1-a (1960). In all, four instances of utilization
of a nest-site a second time were recorded. Two-a (1960) and
2-d (1960) were built in the same fork; 1-c (1960) and 1-h (1960)
were in the same tree, but not the same fork. It should be mentioned
that 1-a (1960) and 2-a (1960) were abortive attempts that
did not progress beyond the suspension apparatus. Nice (1929:16)
recorded a similar instance of the re-use of a nest tree, but different
forks were used.
Re-use of an exact nest-site would ordinarily be impossible if
the initial attempt were not abortive, because the presence of a
completed nest would pose problems in construction with which
the birds would probably be unable to cope. (A report by Morse
in Bent, 1950:256 of a double nest indicates that this may not always
be true. At the time of discovery one nest contained two eggs
and the other nest contained young.) Since nests are used only
once there would be no tendency to adopt the old nest. However,
abortive nests, usually little more than a few strands of nesting
material secured to the fork, might stimulate the birds to continue
building. Re-use of a single nest-site in 15.8 per cent of 38 nests
built in 1960 seems to be more than fortuitous circumstance. This
re-use may have physiological benefits in conjunction with apportionment
of energy for other nesting activities, because rapid location
of a nest-site would mean that energy normally expended in
searching and selecting could be rechanneled for actual construction.
In each of the instances of rebuilding, the new nest was
[Pg 274]
begun on the same day that the previous nest was abandoned.
The re-nesting of pair 9 (1960) is worthy of note. These birds
were established in the elm thicket on Clark land. Elm was by
far the most abundant tree, with dogwood, Osage orange and honey
locust also relatively common. There were only six boxelders in
the territory and yet the four nests built by this pair were placed
in them. This is the only instance of seeming preference.
Building
Nestbuilding by Bell Vireos can be best discussed in terms of
the phases of construction described for the Red-eyed Vireo, Lawrence
(1953:57), which are: (1) construction of the suspension
apparatus, (2) construction of the bag, (3) lining of the bag and
smoothing and polishing of the exterior, and (4) adornment of the
exterior. Red-eyes (Lawrence, 1953:59) may continue adornment
far into the period of incubation. Both the male and female Bell
Vireo have been observed to add spider egg sacs and other silk
to the exterior of the nest as late as the sixth day of incubation.
Nice (1929:16) recorded only the female Bell Vireo building,
but she did recall, from previous studies, having seen males aiding
somewhat. Pitelka and Koestner (1942:102) wrongly concluded
that the female Bell Vireo builds unaided, but Hensley (1950:243)
observed that both sexes participated in nestbuilding, and Mumford
(1952:229) reported two instances of building by both adults.
His description of the activities viewed in mid-May suggest that
they were of the transitional period between the first and second
phases. On the second occasion he recorded both adults building
during the second phase. Since no details accompany this second
observation I assume that it pertained to activity not necessarily
typical of this phase of construction. Whereas both sexes of the
Bell Vireo cooperate in building the nest, only the female Red-eyed
Vireo builds according to Lawrence (1953:56). But Common
(1934:242) saw both Red-eyed Vireos building a nest.
The suspension apparatus is constructed by only the male on the
first day. He punctuates each trip to the nest with song. The single
song phrase is given from three to eight times when the male, carrying
nesting material in his bill, arrives in the tree. Typically, he
alights on several perches within the nest tree before flying to the
nest. He often interrupts his work with several songs; when he
has finished adding a load of material he sings from several perches
[Pg 275]
within the nest tree before departing. The male periodically stops
building to court the female.
In eight hours (494 minutes) of observing the first phase of construction
at five different nests, I saw the female come to the nest
28 times; the male made 95 trips. The female came alone only once,
and brought nesting material ten times, but did not build; on the
other 18 occasions her visits were brief and she usually confined her
activities to an inspection of the nest. Twenty of the visits by the
female were made late in the first phase, marking a gradual transition
to her assumption of building responsibility. (The delay by
the female in beginning to build is puzzling; because all evidence
indicates that she helps select the nest-site, I would expect her to
help with the initial building. There seems to be no clear advantage
in her delay in beginning to build.) The courtship and building
activities of the male plus the presence of a partly completed nest
seem to stimulate the female to commence building. Her visits
become more frequent as construction of the suspension apparatus
nears completion. At a time early in the second day the transition
has taken place, and the female becomes the sole worker.
On May 7, 1960, male 2 (1960), at the time unmated, was observed
as he came upon a nest of the previous year. The nest, after
a year's weathering, suggested in appearance perhaps an early
second-day nest. The bird flew to the nest and tugged and wove
loose strands of grass for three minutes. Before leaving the site, the
bird sang twice from different perches. This observation suggests
that a partly constructed nest can elicit nestbuilding behavior, even
in an unmated male.
The techniques of building by the male consist primarily of laying
pieces of grass or bark across the fork, or along one of its branches,
and then fastening them in place with pieces of animal silk. Once
a "racket" has been formed, spider egg cases and plant down are
emplaced among the fibers. The male employs weaving, twisting,
and pecking motions of the head to emplace material.
As previously indicated, the female is the principal worker in the
second and third phases of construction. The male infrequently
visits the nest, but regularly visits the nest tree. The molding of the
bag is accomplished by piling leaves, grasses and plant down onto
the suspension apparatus. This material is also bound in with animal
silk. As the amount of material accumulates, the female begins to
trample it and gradually the bag takes shape. When trampling is
[Pg 276]
first attempted, the nest often fails to support the female and she
falls through the bottom of the nest. Such an occurrence was observed
on May 23, 1960, on three consecutive trips by female 1
(1960), in constructing nest 1-e (1960). As the bag deepens, additional
strands of grass are added to the wall and woven into place.
The male is extremely attentive during this and the following phase.
He follows the female as she gathers nest-material accompanying both
this activity and her building with rapid song; he may give an average
of seven song phrases per minute. The male brings to the nest a strand
of grass, or some other material, about every twentieth trip. He
frequently inspects the nest and the activities of the female from
perches near the nest. Construction of the bag is ordinarily completed
in the third day.
The third phase, the lining of the interior and the smoothing
of the exterior, involves an additional one and one-half to two days.
Smoothing of the exterior refers to tightening of the grasses woven
into the bag and addition of more animal silk. In lining the nest,
the female stands on one of the branches of the fork and emplaces
one end of a long, thin strand of some relatively stiff piece of
grass or strip of bark. She then jumps into the bag and, while slowly
turning around, pecks it into place, thus coiling the strand neatly
around the interior of the bag.
As previously mentioned, the fourth phase overlaps the periods
of lining, smoothing, egglaying, and incubation. The principal
activity is the addition of white spider egg sacs to the exterior.
The trips are infrequent; but, occasionally, birds will interrupt
an hour of incubation with three or four minutes of active adornment,
during which several trips may be made. Both sexes participate
in this phase.
Gathering of Nesting Material
Nesting materials were gathered anywhere within the territory.
Occasionally materials were collected from within the nest tree,
but usually they were obtained 20 to 200 feet from the nest-site.
On several occasions I observed birds inspecting stems or branches
where bark was frayed. Loose ends are grasped in the beak and
torn free with an upward jerk of the head. Possibly the notch near
the distal end of the upper mandible aids in grasping these strands.
Plant down is first extracted and then rolled into a ball by means
of the beak while held with the feet before being transported to
the nest.
[Pg 277]
Length and Hours of Nestbuilding
As indicated by Nolan (1960:230), accurate determination of the length
of nestbuilding is difficult because of continued adornment and
polishing after the nest is functionally complete. Most of the early
nests for which I have records took from four and one-half to five
days to construct. A four-to five-day period of building is reported
by other observers (Nice, 1929:16; Pitelka and Koestner, 1942:99;
Hensley, 1950:242; Nolan, 1960:230).
One instance of protracted building was recorded. Nest 6-d (1960) was
begun on May 29, 1960, and not completed until nine days later on June
6, 1960. In contrast nest 1-g (1960) begun on May 31, 1960, was
finished three days later on June 2, 1960. Nestbuilding occurs between
the hours of 6:00 a.m. and 5:30 p.m. Heavy rain in the early morning
may delay building.
Abortive Nestbuilding Efforts
Eight of 38 nests started in 1960 were never completed (Table 6).
Six of these abortive attempts were abandoned during, or shortly
after, the completion of the suspension apparatus. Five of these
nests were abandoned because the female did not begin building
following the end of work by the male. The early abandonment of
the other three nests 1-a (1960), 2-c (1960) and 6-e (1960) was
attributable to the interruption of building by the male because of
heavy rain and protracted territorial conflicts. The occurrence of
these abortive nests at any time within the nesting efforts of a single
pair indicates that such attempts are not examples of "false nestbuilding."
Renesting
Renesting after desertion or successful fledging occurs within two
to thirty-six hours. Young were fledged from 1-a (1959) on June
19, 1959, and nest 1-b (1959) was discovered when late in the
second phase of construction on June 22. If the nest was started on
June 20, then renesting took place within 15 hours after fledging.
The Nest
Several authors have described various aspects of the nest of the
Bell Vireo, notably Goss (1891:535); Simmons (in Bent, 1950:256),
Nice (1929:13) and Nolan (1960:230-231). I can add but little to
these descriptions.
The nest itself is a compact structure composed of strips of bark
and strands of grasses that are interwoven and tightly bound with
[Pg 278]
animal silk. The floor of the cup is first lined with a layer of small
leaves and then the entire interior is lined with fine stems or strips
of bark. Feathers are occasionally used to pad the bottom prior to
lining, as are pieces of wool and milkweed down. Nest 2-e (1960)
had been packed with small pieces of soil bearing moss prior to
lining.
Table 6. Abortive Nesting Attempts in May and June of 1960.
| Nest | Length of time worked on | Cause of abandonment |
|---|---|---|
| 1-a | 1 day | Heavy rain |
| 1-h | 2 days | Female failed to build |
| 2-a | 1/2 day | Female failed to build |
| 2-c | 1 day | Protracted territorial dispute |
| 4-a | 1 day | Female failed to build |
| 5-a | 1 day | Female failed to build |
| 6-c | 1 day | Heavy rain |
| 7-a | 2 days | Female failed to build |
Early nests tend to be bulkier, having thicker walls and bottoms
than later efforts. However, nests in May were found to have 16
per cent thicker bottoms and 41 per cent thicker walls than nests
in June (Table 7). Standard nest measurements do not show this
to be so, for the exterior and interior diameters at the rim are governed
by the angle between the two branches of the fork.
Table 7. Dimensions of Nests in May (1960) and June (1960).
| Measurements | May (N 10) | June (N 8) |
|---|---|---|
| External depth | 61.6 mm. | 59.3 mm. |
| Depth of cup | 45.5 mm. | 46.3 mm. |
| Outside diameter | 57.3/55.5 mm. | 54.3/53.5 mm. |
| Inside diameter | 43.4/42.2 mm. | 45.5/43.9 mm. |
| Thickness of forward wall 1 inch below rim | 13.8 mm. | 7.6 mm. |
| Thickness of bottom | 11.3 mm. | 4.6 mm. |
EGGLAYING AND INCUBATION
Egglaying
Egglaying begins the first or second day after completion of the nest.
The female sits in the nest occasionally for periods of five to
twenty-five minutes on the day the nest is completed. This is
interrupted by periods of nest-adornment and foraging; such activities
sometimes keep the female off the nest for several hours. Prior to the
laying of the first egg, only the female is seen on the
[Pg 279]
nest, although the male is often seen sitting quietly within the nest tree a
few feet from the female. The infrequency of the "congested" song and
the alarm (eh-eH-EH) after the inception of "broodiness" indicates
the waning of courtship behavior. As later in incubation only the
"normal" song and the scold are heard.
Eggs are laid early in the morning prior to 5:30 a. m. according to
Nolan (1960:232). The nest is usually left unoccupied for considerable
periods after the first egg is laid, but, on the first day of laying,
both sexes have been observed sitting for brief periods averaging ten
minutes in length. Eggs are laid at one-day intervals until completion
of the clutch. I found incubation to begin with the second egg.
Clutch-size
The average clutch-size of the Bell Vireo in Kansas, based on
thirty-three records, is 3.39 eggs (Table 8). Seasonally, the largest
average clutches are produced in the middle of the breeding season,
that is, in June. Lack (1947:308-309) indicates that in European
passerines the highest seasonal average clutch-sizes likewise occur
in June. The largest average clutch-size in the Bell Vireo is presumably
related to some aspect of the availability of food.
Table 8. Average Numbers of Eggs per Nest (Number of Records in
Parentheses)[F].
| Year | May | June | July | Mean annual clutch-size |
|---|---|---|---|---|
| 1959 1960 | 3.0 (7) 3.3 (6) | 3.2 (12) 3.83 (5) | 3.0 (1) 4.0 (2) | 3.06 3.72 |
| 1959-1960 | 3.17 | 3.52 | 3.5 | 3.39 |
[F]
These data have been supplemented from the literature pertinent to Kansas.
Caution is necessary in determining mean clutch-size in the Bell
Vireo. Eggs occasionally disappear from the nest prior to or during
incubation, without subsequent addition of cowbird eggs. Unfamiliarity
with the history of such a nest on the part of the observer
would lead to an inaccurate determination of clutch-size.
Complete clutches are not replaced with the same regularity as
are nests. I have recorded intervals of six to thirty days between
successive clutches. Successful replacement of clutches is determined
by a number of factors: nest-site, completion of a nest,
weather, predation, and parasitism by the cowbird. The difference
[Pg 280]
between the number of renesting attempts and the successful replacement
of clutches seems to indicate that different physiological
processes are responsible for these two phenomena and that there
is lack of synchrony between them. The development of the ovarian
follicle requires a specific number of days that is not always coincident
with the building of replacement nests. If, in the Bell Vireo,
replacing a nest were solely a responsibility of the female, instead
of involving the male to a considerable extent, it would seem likely
that replacement of nests and the replacement of clutches would
be more closely coordinated.
Incubation
Nice (1954:173) considers the incubation period to be the elapsed time
between the laying of the last egg in a clutch and the hatching of
that egg, when all eggs hatch. My data indicate that, normally,
intensive incubation begins when the second egg is laid and lasts
fourteen days in the Bell Vireo. Nice (1929:99) also considered the
incubation period in this species to be fourteen days but believed it
to commence when the third egg was laid. Pitelka and Koestner
(1942:99) noted that the first and second eggs hatched fourteen days
after laying of the second egg. However, they thought incubation began
with the first egg. This would mean a fifteen-day period for this egg.
All the eggs that Nolan (1960:234) marked hatched in approximately
fourteen days. Eight eggs artificially incubated by Graber (1955:103)
required an average of 15.01 days to hatch. As Van Tyne and Berger
(1959:293) indicate, periods of sitting on the nest, even all night,
do not necessarily mean that incubation has begun, for it has been
demonstrated in several species that birds may sit on an egg without
actually applying heat. My own observations demonstrate that the first
egg may be left unattended for several hours at a time on the day that
it is laid.
The Roles of the Sexes in Incubation
Both the male and female sit on the eggs in the daytime. My study of
histological sections of ventral epidermis indicates that the male
does not possess a brood patch; the increased vascularization typical
of the brood patch in females is not evident in males. But, the male
loses most of the down feathers of the ventral apterium. Also,
according to Bailey (1952:128), the male Warbling Vireo that sits on
the eggs lacks a brood patch.
Bailey (1952:128) suggests that male passerines lacking brood
patches that habitually sit on eggs do not heat the eggs. Thus it
[Pg 281]
cannot be considered true incubation since no increase of temperature in the
eggs is effected by such means. He further notes that it is at night
when eggs are likely to experience a drop in temperature that
embryonic development will be impaired. I have no data directly
pertaining to which sex sits at night, but it is presumably the
female, because she is always seen on the nest early in the morning
and late in the evening.
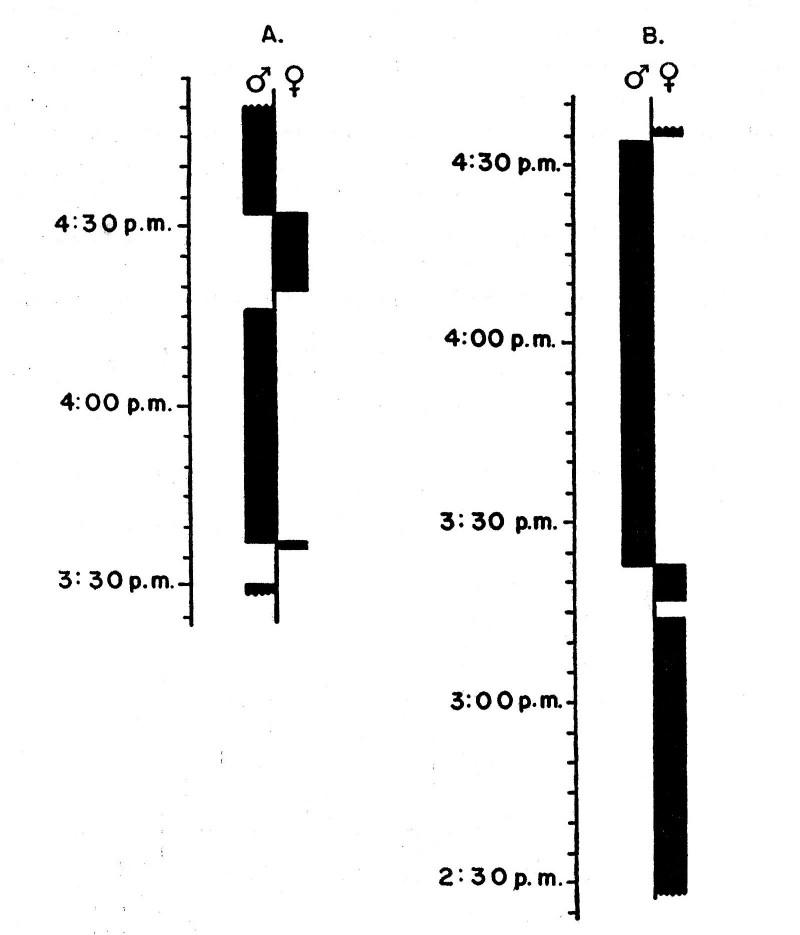
Fig. 4. Comparison of periods of incubation by both
sexes in cold (54° F.) rainy weather (A) and in warm (82° F.) sunny weather (B).
If a highly-vascularized brood patch is essential for true incubation,
then it is surprising that males take regular turns on the nest in
cold, rainy weather. On May 20, 1960, male 3 (1960) sat on the
eggs longer than did the female (fig. 4). The temperature during
[Pg 282]
this hour and a half of incubation was 54° F. One solution to this
problem is supplied by Skutch (1957:74). He indicates that, "the
type of the incubation is determined largely by innate factors, so
that it persists through fairly wide fluctuations in weather, although
it may break down in extreme conditions." Obviously then, in the
example described above, the weather conditions do not qualify as
"extreme." Sitting by the male is certainly functional to some extent
for it relieves the female to forage; furthermore, the eggs are sheltered
from inclement weather and protected from predators. Nolan
(1960:232) suggests similar reasons for incubating by the male
and adds the "conservation of heat supplied to the eggs by the female."
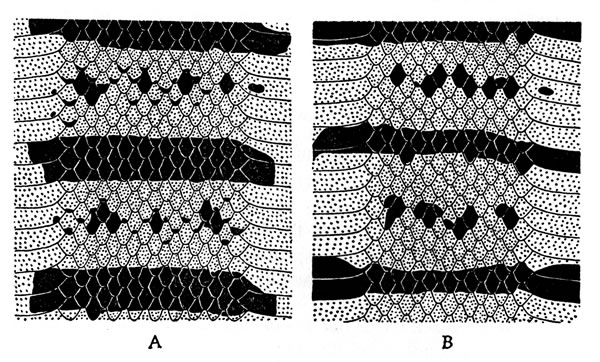
Fig. 5. Daily participation in incubation
as indicated by the sex of the adult on the nest upon approach of the observer.
My data, based on incubation beginning with the second egg, indicate
that the female incubates more often daily than the male (fig. 5). The
male sits on the eggs only occasionally in the morning, but almost as
often as the female in the afternoon. Nolan (1960:233) found that 95.5
per cent of the male's time on the nest and only 40 per cent of the
female's time were attributable to the early hours of the day.
Although I lack data on the critical hours of 5:00 a.m. to 6:59 a.m.,
I have enough observations (20) from 7:00 a.m. to 9:00 a.m. to
indicate that the males sit on the eggs infrequently (3 of 20
instances) in those hours. The discrepancy in the two sets of data,
which may be merely an artifact of sampling techniques, does suggest
two possible alternatives: (1) the male
[Pg 283]
sits on the eggs in the morning and gives the female, who sits on the
eggs throughout the night, an extended rest and an opportunity to
forage; (2) the female continues to sit throughout the morning,
especially during the early hours of daylight, a time of day when the
temperature may still be low enough to impair development of the
embryo.
Relief of Partners in Incubation
Relief of partners involves some ceremony. When the female is
incubating, the male sings several times as he approaches the nest
tree; the female responds with several chees, but otherwise remains
immobile. The male sings several more times upon alighting in the
nest tree whereupon the female chees again and flies directly from
the nest. A few seconds later the male appears at the edge of the
nest and, after inspecting the eggs, hops in and settles upon them.
When the male is sitting he is notably anxious prior to an exchange
with the female, often arising and craning his neck as he surveys the
surrounding vegetation, seemingly searching for his mate. The
singing of the male and the calling of the female serve as signals,
coordinating the exchange.
NESTLING PERIOD
Hatching Sequence
As indicated earlier, hatching normally occurs fourteen days after
the second egg is laid. Hatching of the young was staggered at
three nests under observation. In nest 2-b (1959) the first young
hatched on June 8, 1959, the second on June 10. In 3-b (1959)
one young hatched each day from the 12th through the 14th of
June. In 5-a (1959) two young hatched on June 15, the third on
June 16, and the fourth on June 17. Size of the young differed
notably for about three days as a result of staggered hatching, but
after that day the younger birds tended to catch up in size with
their older brood-mates. The fourth young in nest 5-a (1959)
grew steadily weaker and was missing from the nest on June 23,
1959. Staggered hatching is usually thought to be related to the
availability of food that will insure survival of at least some of the
nestlings when a shortage of food exists. It is doubtful that staggered
hatching has adaptive significance in the Bell Vireo, since
there seems to be no shortage of food for the young. In small
passerines such as the Bell Vireo the principal problem is to insure
fledging as quickly as possible because of the danger from predators.
[Pg 284]
Development of the Nestlings
Young are pinkish at hatching and devoid of visible natal down.
Du Bois (in Wetherbee, 1957:380), inspected day-old nestlings by
means of a magnifying glass and was unable to detect any down.
Nolan (1960:236) also indicates that the young are naked at birth
and that the "body color is between flesh and rufous except
where folds of the straw yellow skin obscure the underlying colors."
The Hutton Vireo (Vireo huttoni) is essentially naked at birth,
save for sparse hairlike down on the head and back (Wetherbee,
1953:380). The Red-eyed Vireo, according to Lawrence (1953:67)
is naked at birth save for a sparse covering of greyish natal
down, on the head, shoulders, and back.
In the Bell Vireo the pterylae darken slightly on the second day
and the color becomes more intense daily until the quills of the
dorsal tracts, the wings, and the tail break from their sheaths on
the sixth day. In Red-eyed Vireos the pterylae darken by the end
of the first day and the quills break through the skin on the fifth
day, erupting from the sheaths by the seventh day (Lawrence,
1953:67).
From the first day the young are able to squeak. Poking a young
bird was sufficient to elicit this sound, phonetically a nasal peek.
The only other vocalization noted throughout the nestling period
was an abbreviated chee.
For the first three days tapping the nest or even movement of it
caused by wind would elicit begging. By the fifth day at nest 2-a
(1959) only vigorous agitation of the branch to which the nest was
[Pg 285]
attached evoked any response. At this nest on June 16, 1959, one
young begged while the other cowered. Cowering is correlated
with opening of the eyes, as the young bird that begged had its
eyes only partly open. Both young cowered on June 19, 1959.
Table 9 summarizes the maturation of the nestling Bell Vireos.
Table 9. Maturation of Nestling Bell Vireos. The First Day That an
Activity Was Observed Is Shown.
| Day of nestling life | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Eyes open | x | ||||||||||
| Feathers erupt | x | ||||||||||
| Sound: Squeak | x | ||||||||||
| Sound: Chee | x | ||||||||||
| Begging | x | ||||||||||
| Cowering | x | ||||||||||
| Head scratching and Preening | x | ||||||||||
| Hopping to rim of nest | x | ||||||||||
| Fledging | x[G] | ||||||||||
[G]
This is the commonest fledging day.
Parental Behavior
No eggshells were found in nests on the days of hatching. Presumably
they had been removed by the parents. Nolan (1960:234) indicates
immediate disposition of the eggshell after hatching. Lawrence
(1953:62) suggests that conspicuous removal of eggshells by the female
Red-eyed Vireo informs the male that the young have hatched.
Both sexes brood and the exchange of partners resembles that
described for the incubation period. Decrease in brooding in the
daytime begins about the sixth day of nestling life. Nolan (1960:235)
reports a sharp decrease in brooding when the oldest nestlings
are seven days old. Brooding decreases notably on the sixth day
of nestling life in the Red-eyed Vireo (Lawrence, 1953:62). Nice
(1929:17), Hensley (1950:244), and Nolan (1960:235) report that
the female Bell Vireo assumes a slightly greater role in brooding
than the male.
Apparent sun-shading was noted at nest 3-b (1959) at 2:00 p.m. on June
17, 1959, on the fifth day of the nestling period. The nest contained
three young. An adult flew to the nest; while standing on its rim the
bird dipped its head into the nest six times, afterward appeared to be
eating a fecal sac, than shifted position to the unattached portion of
the rim, gaped three times, thereupon spread its wings, and sat
motionless 35 minutes. In this attitude it formed an effective shield
sheltering the young from direct sunlight penetrating the thin foliage
of the honey locust in which the nest was situated. The temperature at
this time was 95° F., but the sky was partly cloudy. By 2:30 p.m. the
sky had become overcast and the sun passed behind a cloud. Although
sunlight no longer fell directly upon the nest, the bird remained in
the shielding posture for another five minutes before flying from its
perch. Sun-shading was not observed at either of the other nests
containing young; dense overhead vegetation protected those nests.
Sun-shading has been noted in other species where the nest was poorly
protected from the sun. Lawrence (1953:62) observed this behavior at
two Red-eyed Vireo nests in conifers. The "sun-shield" posture of the
Bell Vireo does not correspond to any of the sunning postures
described by Hauser (1957).
[Pg 286]
Feeding of the Nestlings
Both sexes fed the young, and presumably began shortly after the
first nestling hatched. My data indicate that the female does more
feeding than the male (Table 10); in about eight hours of observation
a total of 67 morsels were brought, 43 by the female and 24 by
the male, for an average of once every 7.6 minutes. Nice (1929:17),
however, observed a male to bring food 53 times as compared to
21 visits by the female. In five and one-half hours of watching,
meals were brought once every 4.9 minutes. Du Bois (in Bent,
1950:257) recorded seven trips in an hour and forty minutes, or one
every fourteen minutes.
At three nests containing young the adults were sometimes silent
and sometimes vocal on their approach. The female often emitted
a subdued chee which, coupled with the vibration of the nest caused
by her arrival, elicited begging behavior from the young. None of
the males was heard to utter such a call, but I have the impression
that they often did call although I failed to hear the sounds. The
males did, on occasion, sing several songs as they approached, even
with food held in their beaks. Such singing elicited begging from
the nestlings. Once the eyes of the young were open they often
began begging when a silent adult was within two or three feet of
the nest; begging behavior probably is elicited by tactile, auditory
or visual stimuli in that order, or, as the nestling period proceeds,
by any combination of these stimuli.
Table 10. Feeding of the Nestlings.
| Day of nestling period | Length of observation | Adult involved | |
|---|---|---|---|
| Male | Female | ||
| 1 | 30 min. | 3 | 5 |
| 2 | 60 min. | 1 | 4 |
| 3 | 60 min. | 2 | 5 |
| 4 | 30 min. | 1 | 4 |
| 7 | 60 min. | 4 | 7 |
| 2 | 60 min. | 3 | 3 |
| 6 | 60 min. | 3 | 6 |
| 7 | 30 min. | 3 | 3 |
| 9 | 60 min. | 4 | 6 |
| Totals | 510 min. | 24 | 43 |
Not all trips made by parents resulted in successful feeding of
young; some visits seemed to be purely for inspecting the young.
[Pg 287]
On other occasions the adults experienced difficulty in transferring
food to the young, and, thus thwarted, would themselves eat the
food. Nice (1929:17) estimated that from five to twelve of a total
of seventy-five meals were eaten by adults.
Nest Sanitation
Both parents regularly removed fecal sacs from the nest, eating
them for the first five days and thereafter carrying them off and
presumably dropping them. It is doubtful that fecal sacs were
actively removed in the last two days of nestling life as the bottoms
of nests from which young flew away were invariably covered with
excrement.
On several occasions a parent brought food to the nest and then
remained perched on the rim alternately peering into the nest and
then preening. Once bill swiping was observed and another time
an adult male sang once. The adult remained at the nest from
twenty seconds to a full minute.
Fledging
Eight young were fledged from the four nests in 1959. The
nestling period lasted from nine to twelve days. Human interference
may have been largely responsible for the fledging of the
young at nine days. Pitelka and Koestner (1942:100) found nestling
life to last eleven days. Nolan (1960:235) reports nestling periods
varying from 10.5 to 12 days. The young Red-eyed Vireo is ready
to leave the nest at ten days but often remains an additional day
before departing (Lawrence, 1953:68).
The oldest nestling at nest 2-a (1959) hopped out on June 17,
1959, when I disturbed the parents. On this date the juvenal
plumage was only partly developed and the young bird was incapable
of flight. By the tenth day of nestling life the young in all
the nests were observed to hop to the rim, flutter their wings, hop
back into the nest and also to preen and scratch their heads. The
young at fledging are usually completely feathered, but have notably
short tails and relatively short, stubby wings. According to Ridgeway
(1904:205) the juvenal plumage is much like that of the adult.
Nest Parasites
Pitelka and Koestner (1942:103) found that incubating adults and later
the young suffered infestation of the northern fowl mite,
Ornithonyseus sylviarum. Nolan (1960:241) reports a heavy
infestation of this mite at four nests. Unidentified mites were noted
at four nests in my study area in 1959. Incubating adults were
[Pg 288]
observed to peck at their breasts and scapulars from the eleventh
through the fourteenth day of incubation. Serious infestations were
not noted at the nests until the ninth day of nestling life. At this
time the young were observed to scratch their heads and peck at their
breasts, scapulars, and the base of their tails. On the day of
fledging the nests were a seething mass of crawling mites; the mites
also extended well up the branches to which the nests were attached.
Nest 1-a (1959), which was discovered on June 18, 1959, presumably on
the day after fledging, was densely covered with mites. Some mites
were still crawling on this nest on June 20, 1959.
FLEDGLING LIFE
On June 20, 1959 I located one young 80 feet northeast of nest 2-a
(1959), about five hours after it had left the nest. One parent was
observed to feed it once. No young were seen thereafter from this or
any other nest. Extreme agitation on the part of one or both parents
on several occasions shortly thereafter, however, suggested the
proximity of the young. Search in the immediate vicinity on each of
these occasions proved fruitless. Three days after fledging their
young, pair 2 (1959) was primarily occupied with courtship activities.
Pair 1 (1959) was involved in courtship and nestbuilding one and
one-half days after the apparent fledging of their young. Nolan
(1960:238) indicates that the young remain within the territory and
perhaps are fed by the parents up until an age of about 40 days.
Sutton (1949:25) and Lawrence (1953:68) present contradictory reports
on fledgling-parent relationships in the Red-eyed Vireo. Sutton
concluded that the young quickly took leave of their parents whereas
Lawrence reported a young bird being fed 35 days after fledging.
Second Broods
The curve based on 66 nesting records of the Bell Vireo representing
the breeding activity in northeastern Kansas demonstrates
a tendency toward double-broodedness (fig. 6). The peak of the
breeding season is from May 20 to June 20. The large number
(20) of replacement nests built in late May of 1960 tends to distort
the curve of the breeding data; a second peak about 35 days after
the first is evident.
I am of the opinion that the vast majority of vireos are single-brooded
solely by virtue of the limited success of early nesting
efforts, and that in "good" years most pairs would be double-brooded.
[Pg 289]
Each of the four pairs that successfully raised one brood
in my study area in 1959 renested within a day or two after the
fledging of the young. I do not know the fate of these nests. Nolan
(1960:237) reports at least one instance of a second brood in the
course of his study. Nolan (op. cit.) notes that the literature, in
general, indicates that vireos are double-brooded, but that his
evidence, mentioned previously, is the only evidence based on
banded birds.
Fig. 6. Breeding season in northeastern Kansas based on
the number of completed clutches in each 10-day period from May through July.
REPRODUCTIVE SUCCESS
Only four nests were successful; all of these were observed in
1959. The principal external factors responsible for nesting failure
were severe weather, predation, parasitism by Brown-headed Cowbirds
(Molothrus ater) and human interference (Table 11).
In late winter and early spring of 1960 heavy snow, continuously
at a depth of at least 10 inches, covered most of the Mid-west from
February 20 through March 20. Consequently, the growing season
was some two weeks behind that of 1959. Of all the species in the
[Pg 290]
study area, the Bell Vireo is the most dependent on dense foliage
for cover and concealment for its nests. Consequently the tardiness
of the season seemingly negatively influenced reproductive success
of this more than any other species of bird in the study area.
Behavior
Several aspects of the behavior of the Bell Vireo tend to contribute
to nesting failure. They include:
1. Nest-site. Nests are occasionally suspended from exposed
branches. Occurrences of this sort suggest that the dimensions of
the fork are more important in the choice of a site than availability
of cover.
2. Song. The loud, continuous song of the male during nestbuilding
alerts cowbirds and predators to the presence of a nest.
The incongruous habits of the male of singing in the nest tree and
while sitting on the nest may facilitate location by some enemies,
particularly cowbirds.
Table 11. Egg Mortality in Bell Vireos.
| Mortality agents | N[H] | Eggs (N-29) 1959 Per cent | N | 1960 Per cent |
|---|---|---|---|---|
| Predation | 4 | 13.8 | 5 | 10 |
| Weather | 2 | 6.9 | 8 | 16 |
| Cowbird | 14 | 48.3 | 37 | 74 |
| Totals | 20 | 69[I] | 50 | 100 |
[H]
Number of eggs out of the total number laid lost to mortality agents.
[I]
In 1959 nine eggs were successful (ultimately gave rise to fledglings).
I am not fully convinced that song from the nest is simply a
"foolish" habit, since snakes, the principal predators with which
this species has to contend, are deaf. My own field observations
and the circumstances of the innumerable instances recorded in the
literature of male vireos singing from the nest suggest that this is a
function of the proximity of the observer. As mentioned elsewhere,
vocal threat is the initial as well as the primary means by which
territory is maintained. Song from the nest evoked by an enemy
also serves to alert the female to danger.
3. Flushing. The Bell Vireo normally relies upon cryptic behavior
to avoid detection at the nest. Most sitting birds, especially
the females, either flush silently when an enemy is about forty feet
[Pg 291]
from the nest or remain sitting upon the nest tenaciously, refusing
to flush even when touched or picked up. Some birds flushed at
intermediate distances of from three to fifteen feet. In so doing
they revealed the location of their nests. Since none of these
"intermediate flushers" enjoyed nesting success there is possibly
some correlation between these two factors.
Predation
Several complete clutches being incubated disappeared from
nests that were unharmed. Absence of eggshells in the vicinity suggests
predation by snakes.
On May 25, 1960, I found a Peromyscus climbing toward nest 1-a
(1960). The mouse moved to within two inches of the nest whereupon
I removed the mouse. Such small rodents constitute another
potential source of predation.
Cowbird Parasitism
In this study the failure of 12 of 35 nests can be directly attributed
to cowbird interference. It is well established that the incidence
of cowbird parasitism of Bell Vireo nests is high (Friedmann,
1929:237; Bent, 1950:260-261). Nolan (1960:240) found only one
nest of eight studied to be parasitized by cowbirds. He indicates
that this is surprising in view of the heavy molestation of the Prairie
Warbler (Dendroica discolor) in the same region. A possible
explanation of this phenomenon seems to lie in the much greater
abundance of the Prairie Warbler in comparison to that of the
Bell Vireo. In my study area the incidence of cowbird parasitism
on Bell Vireos in 1959 and 1960 greatly exceeded that of all other
nesting species that were parasitized (Table 12).
As indicated previously, the female Bell Vireo leaves the nest
unoccupied several hours at a time in the transition period between
completion of the nest and the start of egglaying. Such behavior
early in the morning certainly would facilitate deposition of cowbird
eggs. Early in the nesting period the mere presence of a
cowbird egg in the nest prior to the laying of the host's first egg
leads to abandonment of the nest. This seems to be correlated
with the relative strength of the nesting tendency; anyhow cowbird
eggs laid in later nests prior to the appearance of the host's own
eggs did not cause the nesting birds to desert. The Bell Vireo does
abandon the nest when all but one of its own eggs have been removed
by the cowbird. Mumford (1952:232) records the removal of a cowbird
egg by the host birds and I recorded a similar instance
[Pg 292]
involving nest 2-b (1960). On May 14, 1960, I found one punctured cowbird egg on
the ground about 10 feet west of this nest. Occasionally a cowbird egg
is buried beneath the lining of a nest. Mumford (1952:23) observed
this in mid-May in 1951 and I observed pair 8 (1960) actively covering
with building material a cowbird egg on July 5, 1960. Covering a
cowbird egg constitutes effective removal. Since the egg cannot be
turned, an adhesion develops.
Table 12. Incidence of Cowbird Parasitism of the Bell Vireo Compared
With Other Passerines in the Study Area in 1959 and 1960.
| Bell Vireo | Other passerines | |
|---|---|---|
| Total nests examined containing at least one host egg | 35 | 43 |
| Total nests parasitized | 24 | 14 |
| Total number of cowbird eggs | 33 | 23 |
| Per cent of nests parasitized | 68.6 | 32.6 |
| Total number of cowbird eggs per nest | .94 | .54 |
The percentage of cowbird eggs hatched in relation to the number laid
is relatively low. For instance, Mumford (1952:231) has only one
record of a young cowbird successfully raised by a Bell Vireo. The
data available in Bent (1950:260-261) also indicate that the
percentage of cowbird eggs hatched is small. The Bell Vireo is less
tolerant of cowbird parasitism than are many of the species so
victimized, but is not so intolerant as the Robin, Catbird, and the
Yellow-breasted Chat (Friedmann, 1929:193).
SUMMARY
1. The behavior of a small population of Bell Vireos was studied
in the spring and summer of 1959 and again in 1960 in Douglas
County, Kansas, and results are compared with previous studies
elsewhere.
2. The Bell Vireo sings more often daily and throughout the
nesting season than do the majority of its avian nesting associates.
Six types of vocalizations are readily distinguishable in the field:
primary song, courtship song, distress call, alarm note, specialized
male call note or zip, and the generalized call note or chee.
3. Territories are established in early May and occupied throughout
the breeding season and post-breeding season. The average
[Pg 293]
size of the territories in 1960 was 1.25 acres. Shifting of territorial
boundaries occasionally occurs after nesting attempts.
4. Territory is maintained primarily by song, but at least five
aggressive displays are manifest in the early phases of territorial
establishment. These include: (a) vocal threat, (b) head-forward
threat, (c) wing-flicking and sub-maximal tail-fanning, (d) ruffling
and maximum tail-fanning, and (e) supplanting attack.
5. The precise mechanism of pair-formation in the Bell Vireo
is not known. Early courtship activities are characteristically violent
affairs. Absence of sexual dimorphism suggests that behavioral
criteria are used by the birds in sex-recognition; the male is dominant
and the female is subordinate.
6. The principal displays associated with courtship include:
greeting ceremonies, "pouncing," "leap-flutter," pre- and post-copulatory
displays, and the posture, copulation. The marked similarity
between elements of courtship display and aggressive display suggests
common origin or the derivation of one from the other.
7. The nest-site probably is selected by the female. Nests are
suspended from lateral or terminal forks about 2 feet 3 inches high
in small trees and shrubs averaging 11 feet 2 inches in height.
8. Nestbuilding is intimately associated with courtship and is a
responsibility of both sexes. The male builds the suspension apparatus
and the female constructs and lines the bag. Both sexes
participate in adorning the exterior. Construction lasts from four
and one-half to five days.
9. The nest is compact, pendant, and composed of strips of bark
and strands of grasses that are interwoven and tightly bound with
animal silk. Nests built in May are bulkier than those constructed
later in the season.
10. Egglaying begins on the first or second day after the nest
is completed. The eggs are deposited early in the morning. The
average clutch-size of the Bell Vireo in Kansas is 3.39 eggs.
11. Both sexes sit on the eggs, but only the female truly incubates
because the male lacks a brood patch. Incubation lasts fourteen
days.
12. The Bell Vireo is double-brooded in "good" years.
13. Nesting failure resulted from severe weather, predation,
parasitism by cowbirds, and human interference. Behavior that
contributes to nesting failure is selection of an unfavorable nest-site,
singing on and near the nest, and the tendency to flush from the nest
in view of potential enemies.
[Pg 294]
LITERATURE CITED
American Ornithologists' Union
1957. Check-list of North American birds. Fifth ed. Baltimore, The Lord
Baltimore Press, The American Ornithologists' Union, iv + 691 pp.
Andrew, R. J.
1956. Intention movements of flight in certain passerines, and their use
in systematics. Behaviour, 10:79-204.
Bailey, R. E.
1952. The incubation patch of passerine birds. Condor, 54:121-136.
Bennett, W. W.
1917. Bell's Vireo studies (Vireo bellii Aud.). Proc. Iowa Acad. Sci.,
24:285-293.
Bent, A. C.
1950. Life histories of North American wagtails, shrikes, vireos and their
allies. Smithsonian Inst., U. S. Nat. Mus. Bull., 197:vii + 411 pp.,
48 pls.
Bunker, C. D.
1910. Habits of the Black-capt Vireo (Vireo atricapillus). Condor,
12:70-73.
Chapin, E. A.
1925. Food habits of the vireos; a family of insectivorous birds. U. S.
Dept. Agric. Bull., 1355:1-44.
Common, M. A.
1934. Notes on a Red-eyed Vireo's nest. Auk, 51:241-242.
Cooke, W. W.
1909. The migration of vireos. Bird Lore, 11:78-82, 118-120, 165-168.
Fitch, H. S.
1958. Home ranges, territories and seasonal movements of vertebrates of
the Natural History Reservation. Univ. of Kansas Publ. Mus. of
Nat. Hist., 11:3:63-326.
Friedmann, H.
1929. The Cowbirds. Charles C. Thomas, Springfield, Illinois, xviii +
421 pp.
Goss, N. S.
1891. History of the birds of Kansas. Topeka, Geo. W. Crane & Co.
Printers and Binders. 692 pp.
Graber, R. R.
1955. Artificial incubation of some non-galliform eggs. Wilson Bull.,
67:100-109.
Hamilton, T. H.
1958. Adaptive variation in the genus Vireo. Wilson Bull., 70:307-346.
Hauser, D. C.
1957. Some observations on sun-bathing in birds. Wilson Bull., 69:78-90.
1959. Notes on pairing and nestbuilding of mismatched vireos. Wilson
Bull., 71:383-384.
Hensley, Max
1950. Notes on the breeding behavior of the Bell's Vireo. Auk, 67:243-244.
[Pg 295]
Hinde, R. A.
1952. The behaviour of the Great Tit (Parus major) and some other related
species. Leiden: E. J. Brill, x + 201 pp.
1956. The biological significance of the territories of birds, Ibis,
98:340-369.
Hinde, R. A., and Tinbergen, N.
1958. The comparative study of species-specific behavior. In Behavior
and Evolution (Yale University Press, New Haven), pp. 251-268.
Kluijver, H. N.
1951. The population ecology of the great tit, Parus m. major L. Ardea,
39:1-135.
Lack, D.
1947. The significance of clutch-size. Ibis, 89:302-352.
Lawrence, L. de K.
1953. Nesting life and behavior of the Red-eyed Vireo. Can. Field-Nat.,
67:46-77.
Lewis, H. F.
1921. A nesting of the Philadelphia Vireo. Auk, 38:26-44, 185-202.
Linsdale, J. M.
1928. Birds of a limited area in eastern Kansas. The Univ. of Kansas,
Sci. Bull., 18:11:517-626.
Lloyd, W.
1887. Birds of Tom Green and Concho Counties, Texas. Auk, 4:181-193,
289-299.
Morris, D.
1956. The feather postures of birds and the problem of the origin of social
signs. Behaviour, 9:75-113.
Moynihan, M.
1955. Types of hostile display. Auk, 72:247-259.
Mumford, R. E.
1952. Bell's Vireo in Indiana. Wilson Bull., 64:224-233.
Nice, M. M.
1929. The fortunes of a pair of Bell Vireos. Condor, 31:13-20.
1943. Studies in the life history of the song sparrow. II. The behavior
of the song sparrow and other passerines. Trans. Linn. Soc. N.Y., 6:328 pp.
1954. Problems of incubation periods in North American birds. Condor,
56:173-197.
Nolan, V.
1960. Breeding behavior of the Bell Vireo in southern Indiana. Condor,
62:225-244.
Pitelka, F. A.
1959. Numbers, breeding schedule, and territoriality in Pectoral Sandpipers
of northern Alaska. Condor, 61:223-264.
Pitelka, F. A., and Koestner, E. J.
1942. Breeding behavior of Bell's Vireo in Illinois. Wilson Bull.,
54:97-106.
[Pg 296]
Ridgway, R.
1889. The ornithology of Illinois. Illinois State Nat. Hist. Survey, 1:
viii + 520 pp.
1904. The birds of North and Middle America. U.S. Nat. Mus. Bull.,
50, pt. 3; xx + 801 pp.
Skutch, A. F.
1957. The incubation patterns of birds. Ibis, 99:69-93.
Southern, W. E.
1958. Nesting of the Red-eyed Vireo in the Douglas Lake Region, Michigan.
The Jack-Pine Warbler, 36:105-130, 185-207.
Sutton, G. M.
1949. Studies of the nesting birds of the Edwin S. George Reserve. Part 1.
The Vireos. Misc. Pub. Univ. Michigan Mus. Zool., 74:5-36.
Townsend, C. W.
1920. Supplement to the birds of Essex County, Massachusetts. Mem.
Nuttall Orn. Club, No. 5:196 pp.
Tyler, W. M.
1912. A vireo courtship. Bird Lore, 14:229-230.
Van Tyne, J., and Berger, A. J.
1959. Fundamentals of ornithology. John Wiley & Sons, Inc., New York.
xi + 624 pp.
Wetherbee, D. K.
1957. Natal plumages and downy pteryloses of passerine birds of North
America. Bull. Amer. Mus. Nat. Hist., 113:5:339-436.
Transmitted November 8, 1961.
29-1506
UNIVERSITY OF KANSAS PUBLICATIONS
MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain
this series by addressing the Exchange Librarian, University of Kansas
Library, Lawrence, Kansas. Copies for individuals, persons working in
a particular field of study, may be obtained by addressing instead the
Museum of Natural History, University of Kansas, Lawrence, Kansas.
There is no provision for sale of this series by the University
Library, which meets institutional requests, or by the Museum of
Natural History, which meets the requests of individuals. However,
when individuals request copies from the Museum, 25 cents should be
included, for each separate number that is 100 pages or more in
length, for the purpose of defraying the costs of wrapping and
mailing.
* An asterisk designates those numbers of which the Museum's supply
(not the Library's supply) is exhausted. Numbers published to date, in
this series, are as follows:
Vol. 1. Nos. 1-26 and index. Pp. 1-638, 1946-1950.
*Vol. 2. (Complete) Mammals of Washington. By Walter W. Dalquest. Pp.
1-444, 140 figures in text. April 9, 1948.
Vol. 3. *1. The avifauna of Micronesia, its origin, evolution, and
distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June
12, 1951.
*2. A quantitative study of the nocturnal migration of birds. By
George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951.
3. Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp.
473-530, 49 figures in text, 13 tables. October 10, 1951.
4. Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr.,
and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables.
October 10, 1951.
Index. Pp. 651-681.
*Vol 4. (Complete) American weasels. By E. Raymond Hall. Pp. 1-466,
41 plates, 31 figures in text. December 27, 1951.
Vol. 5. Nos. 1-37 and index. Pp. 1-676, 1951-1953.
*Vol 6. (Complete) Mammals of Utah, taxonomy and distribution. By
Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August
10, 1952.
Vol. 7. *1. Mammals of Kansas. By E. Lendell Cockrum. Pp. 1-303, 73
figures in text, 37 tables. August 25, 1952.
2. Ecology of the opossum on a natural area in northeastern Kansas. By
Henry S. Fitch and Lewis L. Sandidge. Pp. 305-338, 5 figures in text.
August 24, 1953.
3. The silky pocket mice (Perognathus flavus) of Mexico. By Rollin H.
Baker. Pp. 339-347, 1 figure in text. February 15, 1954.
4. North American jumping mice (Genus Zapus). By Phillip H. Krutzsch.
Pp. 349-472, 47 figures in text, 4 tables. April 21, 1954.
5. Mammals from Southeastern Alaska. By Rollin H. Baker and James S.
Findley. Pp. 473-477. April 21, 1954.
6. Distribution of Some Nebraskan Mammals. By J. Knox Jones, Jr. Pp.
479-487. April 21, 1954.
7. Subspeciation in the montane meadow mouse, Microtus montanus, in
Wyoming and Colorado. By Sydney Anderson. Pp. 489-506, 2 figures in
text. July 23, 1954.
8. A new subspecies of bat (Myotis velifer) from southeastern
California and Arizona. By Terry A. Vaughan. Pp. 507-512. July 23,
1954.
9. Mammals of the San Gabriel mountains of California. By Terry A.
Vaughan. Pp. 513-582, 1 figure in text, 12 tables. November 15, 1954.
10. A new bat (Genus Pipistrellus) from northeastern Mexico. By Rollin
H. Baker. Pp. 583-586. November 15, 1954.
11. A new subspecies of pocket mouse from Kansas. By E. Raymond Hall.
Pp. 587-590. November 15, 1954.
12. Geographic variation in the pocket gopher, Cratogeomys castanops,
in Coahuila, Mexico. By Robert J. Russell and Rollin H. Baker. Pp.
591-608. March 15, 1955.
13. A new cottontail (Sylvilagus floridanus) from northeastern Mexico.
By Rollin H. Baker. Pp. 609-612. April 8, 1955.
14. Taxonomy and distribution of some American shrews. By James S.
Findley. Pp. 613-618. June 10, 1955.
15. The pigmy woodrat, Neotoma goldmani, its distribution and
systematic position. By Dennis G. Rainey and Rollin H. Baker.
Pp. 619-624. 2 figures in text. June 10, 1955.
Index. Pp. 625-651.
Vol. 8. Nos. 1-10 and index. Pp. 1-675, 1954-1956.
Vol. 9. 1. Speciation of the wandering shrew. By James S. Findley. Pp.
1-68, 18 figures in text. December 10, 1955.
2. Additional records and extension of ranges of mammals from Utah. By
Stephen D. Durrant, M. Raymond Lee, and Richard M. Hansen. Pp. 69-80.
December 10, 1955.
3. A new long-eared myotis (Myotis evotis) from northeastern Mexico.
By Rollin H. Baker and Howard J. Stains. Pp. 81-84. December 10, 1955.
4. Subspeciation in the meadow mouse, Microtus pennsylvanicus, in
Wyoming. By Sydney Anderson. Pp. 85-104, 2 figures in text. May 10,
1956.
5. The condylarth genus Ellipsodon. By Robert W. Wilson. Pp. 105-116,
6 figures in text. May 19, 1956.
6. Additional remains of the multituberculate genus Eucosmodon. By
Robert W. Wilson. Pp. 117-123, 10 figures in text. May 19, 1956.
7. Mammals of Coahuila, Mexico. By Rollin H. Baker. Pp. 125-335, 75
figures in text. June 15, 1956.
8. Comments on the taxonomic status of Apodemus peninsulae, with
description of a new subspecies from North China. By J. Knox Jones,
Jr. Pp. 337-346, 1 figure in text, 1 table. August 15, 1956.
9. Extensions of known ranges of Mexican bats. By Sydney Anderson. Pp.
347-351. August 15, 1956.
10. A new bat (Genus Leptonycteris) from Coahuila. By Howard J.
Stains. Pp. 353-356. January 21, 1957.
11. A new species of pocket gopher (Genus Pappogeomys) from Jalisco,
Mexico. By Robert J. Russell. Pp. 357-361. January 21, 1957.
12. Geographic variation in the pocket gopher, Thomomys bottae, in
Colorado. By Phillip M. Youngman. Pp. 363-387, 7 figures in text.
February 21, 1958.
13. New bog lemming (genus Synaptomys) from Nebraska. By J. Knox
Jones, Jr. Pp. 385-388. May 12, 1958.
14. Pleistocene bats from San Josecito Cave, Nuevo León, México. By J.
Knox Jones, Jr. Pp. 389-396. December 19, 1958.
15. New subspecies of the rodent Baiomys from Central America. By
Robert L. Packard. Pp. 397-404. December 19, 1958.
16. Mammals of the Grand Mesa, Colorado. By Sydney Anderson. Pp.
405-414, 1 figure in text, May 20, 1959.
17. Distribution, variation, and relationships of the montane vole,
Microtus montanus. By Sydney Anderson. Pp. 415-511, 12 figures in
text, 2 tables. August 1, 1959.
18. Conspecificity of two pocket mice, Perognathus goldmani and P.
artus. By E. Raymond Hall and Marilyn Bailey Ogilvie. Pp. 513-518, 1
map. January 14, 1960.
19. Records of harvest mice, Reithrodontomys, from Central America,
with description of a new subspecies from Nicaragua. By Sydney
Anderson and J. Knox Jones, Jr. Pp. 519-529. January 14, 1960.
20. Small carnivores from San Josecito Cave (Pleistocene), Nuevo León,
México. By E. Raymond Hall. Pp. 531-538, 1 figure in text. January 14,
1960.
21. Pleistocene pocket gophers from San Josecito Cave, Nuevo León,
México. By Robert J. Russell. Pp. 539-548, 1 figure in text. January
14, 1960.
22. Review of the insectivores of Korea. By J. Knox Jones, Jr., and
David H. Johnson. Pp. 549-578. February 23, 1960.
23. Speciation and evolution of the pygmy mice, genus Baiomys. By
Robert L. Packard. Pp. 579-670, 4 plates, 12 figures in text. June 16,
1960.
Index. Pp. 671-690.
Vol. 10. 1. Studies of birds killed in nocturnal migration. By
Harrison B. Tordoff and Robert M. Mengel. Pp. 1-44, 6 figures in text,
2 tables. September 12, 1956.
2. Comparative breeding behavior of
Ammospiza caudacuta and A. maritima. By Glen E. Woolfenden. Pp. 45-75,
6 plates, 1 figure. December 20, 1956.
3. The forest habitat of the University of Kansas Natural History
Reservation. By Henry S. Fitch and Ronald R. McGregor. Pp. 77-127, 2
plates, 7 figures in text, 4 tables. December 31, 1956.
4. Aspects of reproduction and development in the prairie vole
(Microtus ochrogaster). By Henry S. Fitch. Pp. 129-161, 8 figures in
text, 4 tables. December 19, 1957.
5. Birds found on the Arctic slope of northern Alaska. By James W.
Bee. Pp. 163-211, plates 9-10, 1 figure in text. March 12, 1958.
6. The wood rats of Colorado: distribution and ecology. By Robert B.
Finley, Jr. Pp. 213-552, 34 plates, 8 figures in text, 35 tables.
November 7, 1958.
7. Home ranges and movements of the eastern cottontail in Kansas. By
Donald W. Janes. Pp. 553-572, 4 plates, 3 figures in text. May 4,
1959.
8. Natural history of the salamander, Aneides hardyi. By Richard F.
Johnston and Gerhard A. Schad. Pp. 573-585. October 8, 1959.
9. A new subspecies of lizard, Cnemidophorus sacki, from Michoacán,
México. By William E. Duellman, Pp. 587-598, 2 figures in text. May 2,
1960.
10. A taxonomic study of the middle American snake, Pituophis deppei.
By William E. Duellman. Pp. 599-610, 1 plate, 1 figure in text. May 2,
1960.
Index. Pp. 611-626.
Vol. 11. 1. The systematic status of the colubrid snake, Leptodeira
discolor Günther. By William E. Duellman. Pp. 1-9, 4 figures. July 14,
1958.
2. Natural history of the six-lined racerunner, Cnemidophorus
sexlineatus. By Henry S. Fitch. Pp. 11-62, 9 figures, 9 tables.
September 19, 1958.
3. Home ranges, territories, and seasonal movements of vertebrates of
the Natural History Reservation. By Henry S. Fitch. Pp. 63-326, 6
plates, 24 figures in text, 3 tables. December 12, 1958.
4. A new snake of the genus Geophis from Chihuahua, Mexico. By John M.
Legler. Pp. 327-334, 2 figures in text. January 28, 1959.
5. A new tortoise, genus Gopherus, from north-central Mexico. By John
M. Legler. Pp. 335-343. April 24, 1959.
6. Fishes of Chautauqua, Cowley and Elk counties, Kansas. By Artie L.
Metcalf. Pp. 345-400, 2 plates, 2 figures in text, 10 tables. May 6,
1959.
7. Fishes of the Big Blue river basin, Kansas. By W. L. Minckley. Pp.
401-442. 2 plates, 4 figures in text, 5 tables. May 8, 1959.
8. Birds from Coahuila, México. By Emil K. Urban. Pp. 443-516. August
1, 1959.
9. Description of a new softshell turtle from the southeastern United
States. By Robert G. Webb. Pp. 517-525, 2 plates, 1 figure in text.
August 14, 1959.
10. Natural history of the ornate box turtle, Terrapene ornata ornata
Agassiz. By John M. Legler. Pp. 527-669, 16 pls., 29 figures in text.
March 7, 1960.
Index Pp. 671-703.
Vol. 12. 1. Functional morphology of three bats: Eumops, Myotis,
Macrotus. By Terry A. Vaughan. Pp. 1-153, 4 plates, 24 figures in
text. July 8, 1959.
2. The ancestry of modern Amphibia: a review of the evidence. By
Theodore H. Eaton, Jr. Pp. 155-180, 10 figures in text. July 10, 1959.
3. The baculum in microtine rodents. By Sydney Anderson. Pp. 181-216,
49 figures in text. February 19, 1960.
4. A new order of fishlike Amphibia from the Pennsylvanian of Kansas.
By Theodore H. Eaton, Jr., and Peggy Lou Stewart. Pp. 217-240, 12
figures in text. May 2, 1960.
5. Natural history of the bell vireo. By Jon C. Barlow. Pp. 241-296, 6
figures in text. March 7, 1962.
More numbers will appear in volume 12.
Vol. 13. 1. Five natural hybrid combinations in minnows (Cyprinidae).
By Frank B. Cross and W. L. Minckley. Pp. 1-18. June 1, 1960.
2. A distributional study of the amphibians of the Isthmus of
Tehuantepec, México. By William E. Duellman. Pp. 19-72, pls. 1-8, 3
figures in text. August 16, 1960.
3. A new subspecies of the slider turtle (Pseudemys scripta) from
Coahuila, México. By John M. Legler. Pp. 73-84, pls. 9-12, 3 figures
in text. August 16, 1960.
4. Autecology of the copperhead. By Henry S. Fitch. Pp. 85-288, pls.
13-20, 26 figures in text. November 30, 1960.
5. Occurrence of the garter snake, Thamnophis sirtalis, in the Great
Plains and Rocky Mountains. By Henry S. Fitch and T. Paul Maslin. Pp.
289-308, 4 figures in text. February 10, 1961.
6. Fishes of the Wakarusa river in Kansas. By James E. Deacon and
Artie L. Metcalf. Pp. 309-322, 1 figure in text. February 10, 1961.
7. Geographic variation in the North American cyprinid fish. Hybopsis
gracilis. By Leonard J. Olund and Frank B. Cross. Pp. 323-348, pls.
21-24, 2 figures in text. February 10, 1961.
8. Descriptions of two species of frogs, genus Ptychohyla; studies of
American hylid frogs, V. By William E. Duellman. Pp. 349-357, pl. 25,
2 figures in text. April 27, 1961.
9. Fish populations, following a drought, in the Neosho and Marais des
Cygnes rivers of Kansas. By James Everett Deacon. Pp. 359-427, pls.
26-30, 3 figs. August 11, 1961.
10. Recent soft-shelled turtles of North America (family
Trionychidae). By Robert G. Webb. Pp. 429-611, pls. 31-54, 24 figures
in text. February 16, 1962.
Vol. 14. 1. Neotropical bats from western México. By Sydney Anderson.
Pp. 1-8. October 24, 1960.
2. Geographic variation in the harvest mouse, Reithrodontomys
megalotis, on the central Great Plains and in adjacent regions. By J.
Knox Jones, Jr., and B. Morsaloglu. Pp. 9-27, 1 figure in text. July
24, 1961.
3. Mammals of Mesa Verde National Park, Colorado. By Sydney Anderson.
Pp. 29-67, pls. 1 and 2, 3 figures in text. July 24, 1961.
4. A new subspecies of the black myotis (bat) from eastern Mexico. By
E. Raymond Hall and Ticul Alvarez. Pp. 69-72, 1 figure in text.
December 29, 1961.
5. North American yellow bats, "Dasypterus," and a list of the named
kinds of the genus Lasiurus Gray. By E. Raymond Hall and J. Knox
Jones, Jr. Pp. 73-98, 4 figures in text. December 29, 1961.
6. Natural history of the brush mouse (Peromyscus boylii) in Kansas
with description of a new subspecies. By Charles A. Long. Pp. 99-111,
1 figure in text. December 29, 1961.
7. Taxonomic status of some mice of the Peromyscus boylii group in
eastern Mexico, with description of a new subspecies. By Ticul
Alvarez. Pp. 113-120, 1 figure in text. December 29, 1961.
8. A new subspecies of ground squirrel (Spermophilus spilosoma) from
Tamaulipas, Mexico. By Ticul Alvarez. Pp. 121-124. March 7, 1962.
9. Taxonomic status of the free-tailed bat, Tadarida yucatanica
Miller. By J. Knox Jones, Jr., and Ticul Alvarez. Pp. 125-133, 1
figure in text. March 7, 1962.
More numbers will appear in volume 14.
Vol. 15. 1. The amphibians and reptiles of Michoacán, México. By
William E. Duellman. Pp. 1-148, pls. 1-6, 11 figures in text. December
20, 1961.
2. Some reptiles and amphibians from Korea. By Robert G. Webb, J. Knox
Jones, Jr., and George W. Byers. Pp. 149-173. January 31, 1962.
3. A new species of frog (Genus Tomodactylus) from western México. By
Robert G. Webb. Pp. 175-181, 1 figure in text. March 7, 1962.
More numbers will appear in volume 15.
Featured Books

Mary Louise
L. Frank Baum
lid black; /* a thin black line border.. */ padding: 6px; /* ..spaced a bit out from the gr...

Cliges: A Romance
active 12th century de Troyes Chrétien
een the Breton romance and the psychological-analytical novelof our own day.Whence comes this amazin...

Ecological Studies of the Timber Wolf in Northeastern Minnesota
L. David Mech, Louis Daniel Frenzel, Robert R. Ream, John W. Winship, and P. D. Karns
th Central Forest Experiment StationD. B. King, DirectorForest Service—U.S. Department of Agricult...

The People of the Mist
H. Rider Haggard
T ARGUMENT XXXV. BE NOBLE OR BE BASE XXXVI. HOW OTTER CAME BACK XXXVII. “I AM REPAID, QUEEN” XXX...

Poor and Proud; Or, The Fortunes of Katy Redburn: A Story for Young Folks
Oliver Optic
rations the critic may decide I have perpetrated in this volume,I have made the success of Katy Redb...

The Dash for Khartoum: A Tale of the Nile Expedition
G. A. Henty
orge for England: A Tale of Cressy and Poitiers. 5s.By Pike and Dyke: A Tale of the Rise of the Dutc...

The Inventions of the Idiot
John Kendrick Bangs
n plans for the amelioration of the condition of the civilized."The trials of the barbarian are real...

Star Hunter
Andre Norton
fering a counterstroke of indifference in what he had always knownwould be a test of wits.[6] Wass w...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Natural History of the Bell Vireo, Vireo bellii Audubon" :