The Project Gutenberg eBook of Metabolic Adaptation to Climate and Distribution of the Raccoon Procyon Lotor and Other Procyonidae
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Metabolic Adaptation to Climate and Distribution of the Raccoon Procyon Lotor and Other Procyonidae
Author: John N. Mugaas
Kathleen P. Mahlke-Johnson
John Seidensticker
Release date: May 5, 2011 [eBook #36036]
Language: English
Credits: Produced by Colin Bell, Tom Cosmas, Joseph Cooper and the
Online Distributed Proofreading Team at https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK METABOLIC ADAPTATION TO CLIMATE AND DISTRIBUTION OF THE RACCOON PROCYON LOTOR AND OTHER PROCYONIDAE ***
[Cover]

[Pg i]
and Distribution of the Raccoon
Procyon lotor and Other Procyonidae
and Kathleen P. Mahlke-Johnson

SMITHSONIAN INSTITUTION PRESS
Washington, D.C.
1993
[Pg ii]
Mugaas, J. N., J. Seidensticker, and K. Mahlke-Johnson. Metabolic Adaptation to Climate and
Distribution of the Raccoon Procyon lotor and Other Procyonidae. Smithsonian Contributions
to Zoology, number 542, 34 pages, 8 figures, 12 tables, 1993.—Although the family
Procyonidae is largely a Neotropical group, the North American raccoon, Procyon lotor, is more
versatile in its use of climate, and it is found in nearly every habitat from Panama to 60°N in
Canada. We hypothesized that most contemporary procyonids have remained in tropic and
subtropic climates because they have retained the metabolic characteristics of their
warm-adapted ancestors, whereas Procyon lotor evolved a different set of adaptations that have
enabled it to generalize its use of habitats and climates. To test this hypothesis we compared
Procyon lotor with several other procyonids (Bassariscus astutus, Nasua nasua, Nasua narica,
Procyon cancrivorus, and Potos flavus) with respect to (1) basal metabolic rate (Ḣb), (2)
minimum wet thermal conductance (Cmw), (3) diversity of diet (Dd), (4) intrinsic rate of natural
increase (rmax), and, where possible, (5) capacity for evaporative cooling (Ec). We measured
basal and thermoregulatory metabolism, evaporative water loss, and body temperature of both
sexes of Procyon lotor from north central Virginia, in summer and winter. Metabolic data for
other procyonids were from literature, as were dietary and reproductive data for all species.
Procyon lotor differed from other procyonids in all five variables. (1) Procyon lotor's mass
specific Ḣb (0.46 mL O2·g-1·h-1) was 1.45 to 1.86 times greater than values for other procyonids.
(2) Because of its annual molt, Procyon lotor's Cmw was about 49% higher in summer than
winter, 0.0256 and 0.0172 mL O2·g-1·h-1·°C-1, respectively. The ratio of measured to predicted
Cmw for Procyon lotor in winter (1.15) was similar to values calculated for Potos flavus (1.02)
and Procyon cancrivorus (1.25). Values for other procyonids were higher than this, but less than
the value for Procyon lotor (1.76) in summer. On a mass specific basis, Bassariscus astutus had
the lowest Cmw with a ratio of 0.85. (3) Procyon lotor utilized three times as many food
categories as Procyon cancrivorus, Nasua nasua, and Bassariscus astutus; about two times as
many as Nasua narica; and nine times as many as Potos flavus. (4) Intrinsic rate of natural
increase correlated positively with Ḣb. Procyon lotor had the highest rmax (2.52 of expected) and
Potos flavus the lowest (0.48 of expected). The other procyonids examined also had low Ḣb, but
their rmax's were higher than predicted (1.11-1.32 of expected). Early age of first female
reproduction, fairly large litter size, long life span, high-quality diet, and, in one case, female
social organization all compensated for low Ḣb and elevated rmax. (5) Although data on the
capacity for evaporative cooling were incomplete, this variable appeared to be best developed in
Procyon lotor and Bassariscus astutus, the two species that have been most successful at
including temperate climates in their distributions.
These five variables are functionally interrelated, and have co-evolved in each species to form
a unique adaptive unit that regulates body temperature and energy balance throughout each
annual cycle. The first four variables were converted into normalized dimensionless numbers,
which were used to derive a composite score that represented each species' adaptive unit.
Procyon lotor had the highest composite score (1.47) and Potos flavus the lowest (0.39). Scores
for the other procyonids were intermediate to these extremes (0.64-0.79). There was a positive
correlation between the number of climates a species occupies and the magnitude of its
composite score. Linear regression of this relationship indicated that 89% of the variance in
climatic distribution was attributed to the composite scores. Differences in metabolic adaptation,
therefore, have played a role in delimiting climatic distribution of these species.
It was clear that Procyon lotor differed from the other procyonids with respect to
thermoregulatory ability, diet, and reproductive potential. These differences have enabled it to
become a highly successful climate generalist, and its evolution of an Ḣb that is higher than the
procyonid norm appears to be the cornerstone of its success.
Official publication date is handstamped in a limited number of initial copies and is
recorded in the Institution's annual report, Smithsonian Year. Series cover design: The coral
Montastrea cavernosa (Linnaeus).
Library of Congress Cataloging-in-Publication Data
Mugaas, John N.
Metabolic adaptation to climate and distribution of the raccoon Procyon lotor and other Procyonidae / John N. Mugaas,
John Seidensticker, and Kathleen P. Mahlke-Johnson.
p. cm.—(Smithsonian contributions to zoology; no. 542)
Includes bibliographical references (p. )
1. Raccoons-Metabolism-Climatic factors. 2. Procyonidae-Metabolism-Climatic factors. 3. Raccoons-Geographical
distribution. 4. Procyonidae-Geographical distribution. I. Seidensticker, John. II. Mahlke-Johnson,
Kathleen. III. Title. IV. Series.
QL1.S54 no. 542 [QL737.C26] 591 s-dc20 [599.74´443´04542] 93-3119![]() The paper used in this publication meets the minimum requirements of the American
The paper used in this publication meets the minimum requirements of the American
National Standard for Permanence of Paper for Printed Library Materials z39.48—1984.
[Pg iii]
| Page | |
| Introduction | 1 |
| Defining the Problem | 1 |
| Procyonid Origins | 1 |
| Typical Procyonids | 2 |
| The Atypical Procyonid | 3 |
| The Hypothesis | 4 |
| Hypothesis Testing | 4 |
| Adaptive Significance of the Variables | 4 |
| Basal Metabolic Rate and Intrinsic Rate of Natural Increase | 4 |
| Minimum Thermal Conductance | 4 |
| Capacity for Evaporative Cooling | 5 |
| Diet | 5 |
| Experimental Design and Summary | 5 |
| Acknowledgments | 5 |
| Materials and Methods | 6 |
| Live-trapping | 6 |
| Metabolic Studies | 6 |
| Basal and Thermoregulatory Metabolism | 6 |
| Evaporative Water Loss | 7 |
| Body Temperature | 7 |
| Calibrations | 7 |
| Calorimeter | 7 |
| Body Temperature Transmitters | 8 |
| Statistical Methods | 8 |
| Estimating Intrinsic Rate of Natural Increase | 8 |
| Comparison of Adaptive Units | 8 |
| Results | 8 |
| Body Mass | 8 |
| Basal Metabolic Rate | 9 |
| Minimum Thermal Conductance | 9 |
| Evaporative Water Loss | 11 |
| Thermoregulation at Low Temperatures | 12 |
| Body Temperature | 12 |
| Summer | 14 |
| Winter | 14 |
| Thermoregulation at High Temperatures | 16 |
| Body Temperature | 16 |
| Summer | 16 |
| Winter | 16 |
| Daily Cycle of Body Temperature | 16 |
| Discussion | 16 |
| Basal Metabolic Rate | 16 |
| Background | 16 |
| Captive versus Wild Raccoons | 17 |
| Seasonal Metabolism of Raccoons | 17 |
| Comparison of Procyon lotor with Other Procyonids | 17 |
| Influence of Diet on Basal Metabolism[Pg iv] | 18 |
| Background | 18 |
| Food Habits of Procyonids | 18 |
| Food Habits and Basal Metabolism | 19 |
| Summary | 19 |
| Basal Metabolism and Intrinsic Rate of Natural Increase | 19 |
| Background | 19 |
| Procyon lotor | 19 |
| Bassariscus astutus | 19 |
| Nasua narica | 19 |
| Nasua nasua | 20 |
| Procyon cancrivorus | 20 |
| Potos flavus | 20 |
| Summary | 20 |
| Basal Metabolism and Climatic Distribution | 21 |
| Procyon lotor | 21 |
| Other Procyonids | 21 |
| Minimum Thermal Conductance | 21 |
| Background | 21 |
| Effect of Molt on Thermal Conductance | 21 |
| Comparison of Thermal Conductances | 22 |
| Procyon lotor versus Tropical Procyonids | 22 |
| Bassariscus astutus | 22 |
| Thermoregulation and Use of Stored Fat at Low Temperatures | 22 |
| Background | 22 |
| Thermoregulation | 22 |
| Stored Fat | 23 |
| Thermal Model of the Raccoon and Its Den | 23 |
| Metabolic Advantage of the Den | 23 |
| Thermoregulation at High Temperatures | 24 |
| Background | 24 |
| Comparison of Procyonid Responses to Heat Stress | 24 |
| Potos flavus | 24 |
| Nasua nasua and Nasua narica | 24 |
| Bassariscus astutus | 24 |
| Procyon lotor | 24 |
| Procyon cancrivorus | 24 |
| Summary | 24 |
| Composite Scores of Adaptive Units and Geographic Distribution | 25 |
| Evolution of Metabolic Adaptations | 26 |
| Evolution of Low Basal Metabolic Rate | 26 |
| Evolution of High Basal Metabolic Rate | 27 |
| Summary | 28 |
| Appendix: List of Symbols | 29 |
| Literature Cited | 30 |
[Pg 1]
and Distribution of the Raccoon
Procyon lotor and Other Procyonidae
and Kathleen P. Mahlke-Johnson
John N. Mugaas, Department of Physiology, Division of Functional
Biology, West Virginia School of Osteopathic Medicine, Lewisburg,
West Virginia 24901. John Seidensticker and Kathleen P. Mahlke-Johnson,
National Zoological Park, Smithsonian Institution, Washington,
D.C. 20008.
The major carnivore radiations took place about 40 million
years before present (MYBP) in the late Eocene and early
Oligocene (Ewer, 1973:363; Wayne et al., 1989). Between 30
and 40 MYBP, a progenitor split into the ursid and procyonid
lineages, which evolved into present-day bears, pandas, and
raccoons (Wayne et al., 1989). The taxonomic relatedness of
pandas to bears and raccoons has been tested extensively and a
number of authors have summarized current thinking on the
problem (Martin, 1989; Wayne et al., 1989; Wozencraft,
1989a, 1989b; Decker and Wozencraft, 1991). Davis
(1964:322-327) and others (Leone and Wiens, 1956; Todd and
Pressman, 1968; Sarich, 1976; O'Brien et al., 1985) place the
giant panda, Ailuropoda melanoleuca, with the ursids. The
taxonomic status of the red panda, Ailurus fulgens, appears to
be less certain. Some current investigations align the red panda
with bears (Segall, 1943; Todd and Pressman, 1968; Hunt,
1974; Ginsburg, 1982; Wozencraft, 1984:56-110; 1989a),
whereas others place them intermediate to procyonids and
bears (Wurster and Benirschke, 1968; Sarich, 1976; O'Brien et
al., 1985), or in close relationship to the giant panda (Tagle et
al., 1986).
The procyonid radiation took place in North America and
produced forms that were mostly arboreal and omnivorous
(Eisenberg, 1981:122; Martin, 1989). The center of this
diversification occurred in Middle America (Baskin, 1982;
Webb, 1985b) during the Miocene (Darlington, 1963:367;
Webb, 1985b). Fossil procyonids from the late Miocene are
represented in Florida, California, Texas, Nebraska, Kansas,
and South Dakota (Baskin, 1982; Martin, 1989) and include
such genera as Bassariscus, Arctonasua, Cyonasua, Paranasua,
Nasua, and Procyon (Baskin, 1982; Webb, 1985b).
During the Miocene procyonids underwent a modest radiation
within tropical and subtropical climates of North America's
central and middle latitudes. Cyonasua, which has close
affinities to Arctonasua (Baskin, 1982), appears in tropical
South America in the late Miocene and immigrated there either
by rafting across the Bolivar Trough or by island-hopping
through the Antilles archipelagoes (Marshall et al., 1982;
Marshall, 1988). Thus, procyonids were found on both
continents prior to formation of the Panamanian land bridge
(Darlington, 1963:367, 395; Marshall et al., 1982; Marshall,
1988). Origins of Bassaricyon and Potos are obscure but
probably occurred in tropical rainforests of Middle America
(Baskin, 1982; Webb, 1985b). A subsequent Pleistocene
dispersal carried several modern genera (Table 1) across the
Panamanian land bridge into South America (Webb, 1985b).
Bassariscus and Bassaricyon represent the most primitive
genera in Procyoninae and Potosinae subfamilies, respectively
(Table 1; Wozencraft, 1989a; Decker and Wozencraft, 1991).
In the early Tertiary, mid-latitudes of North America were
much warmer than they are now, but not fully tropical, and
temperate deciduous forests, associated with strongly seasonal
climates, occurred only in the far north (Barghoorn, 1953;
Colbert, 1953; Darlington, 1963:589, 590). Major climatic
deteriorations, with their attendant cooling of northern continents,
occurred during the Eo-Oligocene transition, in the
middle Miocene, at the end of the Miocene, and at about 3
MYBP (late Pliocene). This last deterioration corresponds with
closure of the Panamanian isthmus (Berggren, 1982; Webb,
[Pg 2]
1985a). Climatic deterioration went on at an accelerating rate
during the late Tertiary, with glacial conditions developing at
the poles by the mid-Pliocene (Barghoorn, 1953). Therefore,
throughout the Tertiary, as continents cooled, northern climate
zones moved toward the tropics (Barghoorn, 1953; Colbert,
1953; Darlington, 1963:589, 590, 594, 595; Webb, 1985a).
Table 1.—Classification of recent Procyonidae after Wozencraft (1989a) and Decker and Wozencraft (1991). Information in parenthesis indicates general geographic distribution (modified from Kortlucke and Ramirez-Pulido (1982) and Poglayen-Neuwall (1975)): S.A. = South America; C.A. = Central America; M. = Mexico; U.S. = United States; C. = Canada. Lower case letters preceding geographic areas signify north (n), south (s), and west (w).
Order Carnivora Bowdich, 1821
Suborder Caniformia Kretzoi, 1945
Family Procyonidae Gray, 1825
Subfamily Potosinae Trouessart, 1904
Genus Potos E. Geoffroy and G. Cuvier, 1795
P. flavus (S.A., C.A., M.)
Genus Bassaricyon Allen, 1876
B. alleni[A] (S.A.)
B. beddardi[A] (S.A.)
B. gabbii[A] (nS.A., C.A.)
B. lasius[A] (C.A.)
B. pauli[A] (C.A.)
Subfamily Procyoninae Gray, 1825
Genus Bassariscus Coues, 1887
B. astutus (M., wU.S.)
B. sumichrasti (C.A., M.)
Genus Nasua Storr, 1780
N. narica[B] (nS.A., C.A., M., swU.S.)
N. nasua[B] (S.A., sC.A.)
Genus Nasuella Hollister, 1915
N. olivacea (S.A.)
Genus Procyon Storr, 1780
P. cancrivorus (S.A., sC.A.)
P. gloveralleni[C] (Barbados)
P. insularis[C] (Maria Madre Is., Maria Magdalene Is.)
P. lotor[C] (C.A., M., U.S., sC.)
P. maynardi[C] (Bahamas, New Providence Is.)
P. minor[C] (Guadeloupe Is.)
P. pygmaeus[C] (M., Quintana Roo, Cozumel Is.)
[A] The several named forms of Bassaricyon are a single species, Bassaricyon gabbii (Wozencraft, 1989a).
[B] These are considered conspecific in some current taxonomies (Kortlucke and Ramirez-Pulido, 1982); however, the scheme followed here maintains them as separate species (Decker, 1991).
[C] Several named forms of Procyon are a single species, Procyon lotor (Wozencraft, 1989a).
During the late Miocene, late Pliocene, and Pleistocene, the
Bering land bridge between North America and Asia formed
periodically, offering an avenue for dispersal between northern
continents (Darlington, 1963:366; Webb, 1985a). However, by
the late Tertiary, northern continents had cooled to the extent
that climate, with its attendant sharply defined vegetative
zones, became the major factor limiting dispersal by this route
(Darlington, 1963:366; Webb, 1985a). Those Holarctic mammals
that did cross the Bering land bridge in the late Tertiary
were "cold-adapted" species associated with relatively cool,
but not alpine, climates (Darlington, 1963:366; Ewer,
1973:369). Among carnivores this included some canids,
ursids, mustelids, and felids (Darlington, 1963:393-395, 397;
Webb, 1985a). Procyonids, however, did not cross the Bering
land bridge into Asia, and Ewer (1973:369) ascribes this to
their being an "essentially tropical group." Miocene radiation
of procyonids occurred at a time when two of the four major
climatic deteriorations (middle and late Miocene) were taking
place (Webb, 1985a, 1985b). These deteriorations had the
effect of cooling the middle latitudes to the extent that
temperate forest forms began to appear in mid-latitude floras,
along with a rapid influx of herbaceous plants (Barghoorn,
1953). The procyonid radiation did not penetrate beyond these
climatically changing middle latitudes, which implies that
these animals were "warm-adapted," and were, therefore,
physiologically excluded from reaching the Bering land bridge.
Today, three of the six genera and over half of the 18 species
that comprise Procyonidae (Table 1; Wozencraft, 1989b)
remain confined to tropical regions of North and South
America (Hall and Kelson, 1959:878-897; Poglayen-Neuwall,
1975; Kortlucke and Ramirez-Pulido, 1982; Nowak and
Paradiso, 1983:977-985).
McNab (1988a) contends that basal metabolism is a highly
plastic character in evolution, and he has amply shown that
ecologically uniform species are more apt to share common
metabolic rates than taxonomically allied species from drastically
different environments (McNab, 1984a, 1986a, 1986b,
1988a). Procyonids represent a taxonomically allied group that
shared a common ecological situation for millions of years;
consequently, members of this family might be expected to
show some uniformity in their Ḣb. Basal and thermoregulatory
metabolism of several procyonids have been measured:
kinkajou, Potos flavus (Müller and Kulzer, 1977; McNab,
1978a; Müller and Rost, 1983), coatis, Nasua nasua (Chevillard-Hugot
et al., 1980; Mugaas et al., in prep.), and Nasua
narica (Scholander et al., 1950c; Mugaas et al., in prep.),
ringtail, Bassariscus astutus (Chevalier, 1985), and crab-eating
raccoon, Procyon cancrivorus (Scholander et al., 1950c). In
general, these species have Ḣb's that are 40%-80% of the
values predicted for them by the Kleiber (1961:206) equation.
Lower than predicted Ḣb is viewed as an energy-saving
adaptation for procyonids living in relatively stable tropical
climates (Müller and Kulzer, 1977; Chevillard-Hugot et al.,
1980; Müller and Rost, 1983). This implies that lower than
predicted Ḣb is a general procyonid condition and that it
represents a characteristic that evolved in response to the
family's long association with tropical and subtropical forest
environments.[Pg 3]
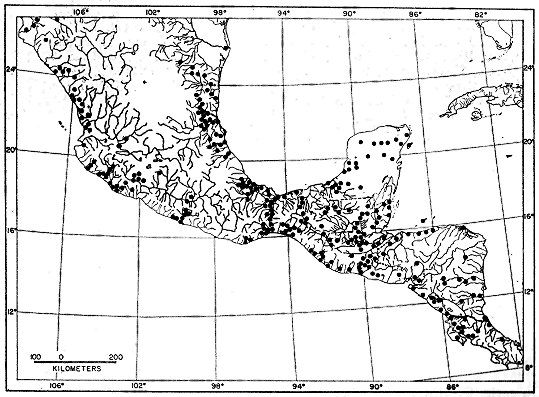
Although most procyonids are found in only tropical to
subtropical climates, the North American raccoon, Procyon
lotor, (Figure 1) has a much broader distribution that extends
from tropical Panama (8°N) to southern Canada. In Alberta,
Canada, its range reaches the edge of the Hudsonian Life Zone
at 60°N (for distribution maps see Hall and Kelson, 1959:878-897,
and Poglayen-Neuwall, 1975). Range extensions and an
increase in numbers have been noted in Canada and in parts of
the United States since the 19th century (Lotze and Anderson,
1979; Kaufmann, 1982; Nowak and Paradiso, 1983:977-985).
Thus, Procyon lotor is more complex ecologically than other
procyonids, particularly when one takes into account its highly
generalized food habits (Hamilton, 1936; Stuewer, 1943;
Stains, 1956:39-51; Greenwood, 1981) and the wide range of
habitat types (forest, prairie, desert, mountain, coastal marsh,
freshwater marsh) and climates (tropical to north temperate) in
which it is successful (Whitney and Underwood, 1952:1; Hall
and Kelson, 1959:885; Lotze and Anderson, 1979; Kaufmann,
1982). On this basis it is clear that Procyon lotor has deviated
from the typical procyonid portrait and has become the
consummate generalist of the Procyonidae.
[Pg 4]Our
general hypothesis was that whereas most contemporary
procyonids have retained the metabolic characteristics of their
warm-adapted ancestors, Procyon lotor possesses a different
set of adaptations, which either evolved as characteristics
unique to this species or were acquired from its ancestral stock.
In either case, its unique adaptations have given Procyon lotor
the physiological flexibility to generalize its use of habitats and
climates and expand its geographic distribution to a much
greater extent than other procyonids.
We tested our hypothesis by comparing Procyon lotor with
several other procyonids (Bassariscus astutus, Nasua nasua,
Nasua narica, Procyon cancrivorus, and Potos flavus) on the
basis of their (1) basal metabolic rate (Ḣb), (2) minimum wet
thermal conductance (Cmw), (3) diversity of diet (Dd), (4)
intrinsic rate of natural increase (rmax), and, when data were
available, (5) capacity for evaporative cooling (Ec). In a genetic
sense each one of these variables is a complex adaptive
characteristic, expression of which is determined by the
interaction of several genes (Prosser, 1986:110-165). Experience
has shown that a given species will express each one of
these variables in a specific manner that is relevant to its mass,
physiology, behavior, and environmental circumstance. Thus,
different expressions of these variables may represent specific
climatic adaptations (Prosser, 1986:16) that have been selected-for
by evolutionary process. Because these variables are
interrelated with respect to regulation of body temperature and
energy balance, they have co-evolved in each species to form
an adaptive unit. For each species, measured and calculated
values for the first four variables were converted into
dimensionless numbers and used to derive a composite score
that represented its adaptive unit. Climatic distributions of
these species were then compared relative to their composite
scores.
Basal metabolic rate represents the minimum energy
required by an animal to maintain basic homeostasis (Lusk,
1917:141; Kleiber, 1932, 1961:251; Benedict, 1938; Brody,
1945:59; Robbins, 1983:105-111). For mammals, Ḣb appears
to be determined by complex interactions between their body
size (Kleiber, 1932, 1961:206; Benedict, 1938; Brody,
1945:368-374; Hemmingsen, 1960:15-36; McNab, 1983b;
Calder, 1987), the climate in which they live (Scholander et al.,
1950c; McNab and Morrison, 1963; Hulbert and Dawson,
1974; Shkolnik and Schmidt-Nielsen, 1976; McNab, 1979a;
Vogel, 1980), their food habits (McNab, 1978a, 1978b, 1980a,
1983a, 1984a, 1986a, 1986b, 1988a, 1989), and their circadian
period (Aschoff and Pohl, 1970; Prothero, 1984). Some species
have higher mass-specific Ḣb than others, and this variation
appears to be tied to ecological circumstances rather than
taxonomic affinities (McNab, 1988a, 1989). Basal metabolic
rate is important ecologically because it serves as a measure of
a species' minimum "obligatory" energy requirement, and
under many circumstances, it represents the largest energy
demand associated with a daily energy budget (King, 1974:38-55;
McNab, 1980a; Mugaas and King, 1981:37-40). Recently
it also has been implicated as a permissive factor with respect
to rmax of mammals (Hennemann, 1983; Lillegraven et al.,
1987; Nicoll and Thompson, 1987; Thompson, 1987) via its
direct effect on their rates of development and fecundity
(McNab, 1980a, 1983a, 1986b; Hennemann, 1983; Schmitz
and Lavigne, 1984; Glazier, 1985a, 1985b). The implication of
this latter point is that those species with higher Ḣb's also have
faster rates of development and greater fecundity and hence
enjoy the competitive advantage of a higher rmax. Basal
metabolism is, therefore, "a highly plastic character in the
course of evolution" (McNab, 1988a:25) that has a profound
influence on each species' life history.
Whole-body resistance to passive heat transfer is equal to
tissue resistance plus coat resistance. Within limits, these
resistances can be altered; tissue resistance can be varied by
changes in blood flow, whereas coat resistance can be changed
by piloerection, molt, and behavior. When whole-body
resistance is maximized (maximum tissue and coat resistances),
passive heat transfer is minimized. The inverse of resistance is
conductance; therefore, maximum whole-body resistance is the
inverse of minimum thermal conductance (Cm). Minimum
thermal conductance is readily derived from metabolic chamber
data, and it is commonly used to describe an animal's
capacity to minimize passive heat transfer. Minimum thermal
conductance interacts with Ḣb and body mass to set the
maximum temperature differential a mammal can maintain
without increasing its basal level of heat production. The low
temperature in this differential is the lower critical temperature
(Tlc).
Mass-specific Cm for mammals is negatively correlated with
body mass (McNab and Morrison, 1963; Herreid and Kessel,
1967; McNab, 1970, 1979b; Bradley and Deavers, 1980;
Aschoff, 1981), and for any given mass its magnitude is 52%
higher during the active, rather than the inactive, phase of the
daily cycle (Aschoff, 1981). However, some mammals have
Cm's that are higher or lower than would be predicted for them
on the basis of body mass and circadian phase. Seasonal
[Pg 5]
variation in Cm (higher values during summer than winter) has
been reported for many northern mammals that experience
large annual variations in air temperature (Scholander et al.,
1950a; Irving et al., 1955; Hart, 1956, 1957; Irving, 1972:165).
Some tropical mammals with very thin fur coats, and others
with nearly hairless bodies, have high Cm's (McNab, 1984a), as
do burrowing mammals (McNab, 1966, 1979b, 1984a) and the
kit fox, Vulpes macrotis (Golightly and Ohmart, 1983). Some
small mammals with low basal metabolic rates tend to have
lower than predicted Cm's: small marsupials (McNab, 1978a),
heteromyid rodents (McNab, 1979a), several ant eaters
(McNab, 1984a), the arctic hare, Lepus arcticus (Wang et al.,
1973), the ringtail, Bassariscus astutus (Chevalier, 1985), and
the fennec, Fennecus zerda (Noll-Banholzer, 1979). Thus, in
spite of its mass dependence, Cm also has been modified during
the course of evolution by selective factors in the environment
and by the animal's own metabolic characteristics.
Latent heat loss occurs as a result of evaporation from the
respiratory tract and through the skin, and except under
conditions of heat stress, it "is a liability in thermal and osmotic
homeostasis" (Calder and King, 1974:302). Ec, defined as the
ratio of evaporative heat lost to metabolic heat produced, can be
used to quantify thermoregulatory effectiveness of evaporative
cooling and to make comparisons of heat tolerance between
species. Thermoregulatory effectiveness of latent heat loss is
not just a function of the rate of evaporative water loss but also
of the rate of metabolic heat production (Lasiewski and
Seymour, 1972). For example, a low metabolic rate minimizes
endogenous heat load and thus conserves water, whereas the
opposite is true of high metabolic rates (Lasiewski and
Seymour, 1972). Some mammals that live in arid regions have
evolved low metabolic rates and thus capitalize on this
relationship to reduce their thermoregulatory water requirement
(McNab and Morrison, 1963; McNab, 1966; MacMillen
and Lee, 1970; Noll-Banholzer, 1979). What is evident,
therefore, is that an animal's capacity for increasing latent heat
loss must evolve together with its Ḣb and Cm in response to
specific environmental demands.
McNab (1986a, 1988a, 1989) demonstrated that, for mammals,
departures of Ḣb from the Kleiber (1961:206) "norm" are
highly correlated with diet and independent of phylogenetic
relationships. McNab's analysis indicates that for mammals
that feed on invertebrates, those species with body mass less
than 100 g have Ḣb's that are equal to or greater than values
predicted by the Kleiber equation, whereas those with body
mass greater than 100 g have metabolic rates that are lower than
predicted. Grazers, vertebrate eaters, nut eaters, and terrestrial
frugivores also have Ḣb's that are equal to or greater than
predicted, whereas insectivorous bats, arboreal folivores,
arboreal frugivores, and terrestrial folivores all have rates that
are lower than predicted. McNab (1986a) found animals with
mixed diets harder to categorize, but in general he predicted
that their Ḣb's would be related to (1) a food item that is
constantly available throughout the year, (2) a food item that is
most available during the worst conditions of the year, or (3) a
mix of foods available during the worst time of the year.
Although these correlations do not establish cause and effect
between food habits and Ḣb, McNab's analysis does make it
clear that the relationship between these variables has very real
consequences for an animal's physiology, ecology, and
evolution.
In this investigation we measured basal and thermoregulatory
metabolism, evaporative water loss, and body temperature
of raccoons from north central Virginia. Measurements were
conducted on both sexes in summer and winter to determine
how season and sex influenced these variables. We then
compared the data for this widely distributed generalist with
data from literature for its ecologically more restricted
relatives. Dietary data for all species were taken from literature,
as were reproductive data for calculation of rmax.
Our analysis demonstrated clear differences between Procyon
lotor and other procyonids with respect to Ḣb, Cmw, Dd,
and rmax. The composite score calculated from these variables
for Procyon lotor was much higher than those derived for other
species, and there was a positive correlation between the
number of climates a species occupies and the magnitude of its
composite score. Data on evaporative water loss, although not
complete for all species, suggested that tropical and subtropical
procyonids have less capacity for evaporative cooling than
Procyon lotor or Bassariscus astutus. It was clear, therefore,
that with respect to its thermal physiology, Procyon lotor
differed markedly from other procyonids, and we contend that
these differences have allowed this species to become a highly
successful climate generalist and to expand its distribution into
many different habitats and climates. Our analysis also
suggested that the cornerstone of Procyon lotor's success as a
climate generalist is its Ḣb, which is higher than the procyonid
norm.
The authors would like to thank John Eisenberg and Devra
Kleiman for their support and encouragement throughout the
study. This investigation was supported by research grants
from the West Virginia School of Osteopathic Medicine
(WVSOM), and Friends of the National Zoo (FONZ). Logistic
support was provided by the National Zoological Park's
Conservation and Research Center (CRC), and the departments
of Mammalogy and Zoological Research. Our ability to
[Pg 6]
conduct physiological research at CRC was made possible by
the thoughtful support and encouragement provided by Chris
Wemmer. His excellent staff at CRC, especially Jack Williams,
Junior Allison, and Red McDaniel, were very helpful in
providing hospitality and logistical support to the senior author
and his family during their various visits to the Center. The
assistance of several people at the National Zoo also is
gratefully acknowledged: Mitch Bush and Lyndsay Phillips not
only provided veterinary support throughout the investigation,
but also performed surgical procedures required to implant
temperature-sensitive radio transmitters in several raccoons;
Olav Oftedal made his laboratory available to us at various
times and loaned us equipment to use at CRC; Miles Roberts
and his staff provided care for our captive raccoons in the
Department of Zoological Research during various parts of the
investigation. Greg Sanders and Ken Halama, supported by
FONZ assistantships, cared for our captive raccoons at CRC,
provided assistance in the laboratory whenever needed, and
were an invaluable source of aid. Their friendship and help is
gratefully acknowledged. Ellen Broudy and Andy Meyer,
supported by WVSOM and a student work study grant,
respectively, provided assistance in the laboratory. David
Brown, John Eisenberg, Mary Etta Hight, Brian McNab, Steve
Thompson, and W. Chris Wozencraft critically reviewed
various phases of the manuscript and provided many helpful
suggestions. We deeply appreciate the work of Jean B.
McConville, whose beneficial editorial suggestions helped us
improve several early versions of the manuscript. We also
gratefully acknowledge Diane M. Tyler, our editor at the
Smithsonian Institution Press, whose expertise helped us mold
the manuscript into its final form. Jill Mellon and Sriyanie
Miththalapa, supported by FONZ traineeships, assisted in
measuring the daily cycle of body temperature in raccoons. The
Virginia Commission of Game and Inland Fisheries gave us
permission to use wild-caught raccoons in this project.
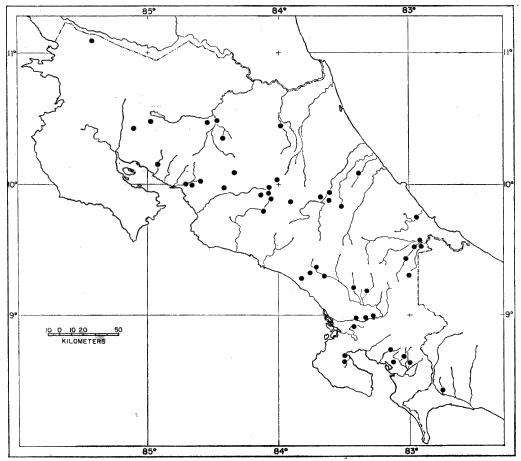
Raccoons were caught from May 1980 through December
1984 on a trapping grid of 30 to 35 stations (one or two "live
traps" per station) that covered about one-third of the National
Zoological Park's Conservation and Research Center (CRC)
near Front Royal, Virginia (Seidensticker et al., 1988; Hallett et
al., 1991). Animals were trapped during 10 consecutive days
each month, and in this five-year interval 407 raccoons were
captured and marked with tattoos and ear tags. All captured
animals were individualized with respect to age, reproductive
status, physical condition, parasite load, and mass and body
dimensions. These data characterized the structure and dynamics
of the raccoon population at CRC and provided information
on the annual cycle of fattening for raccoons in north central
Virginia.
Animals used for metabolic measurements were captured at
CRC about 1.5 km south of the trapping grid and thus were
genetically representative of the area. Six males were captured
and measured during the summer of 1983. These animals were
kept isolated for a week before being measured and were
released later that summer at the site of their capture. The other
seven animals used in our study were from the collection of the
National Zoological Park and all of them had their origins at CRC.
Metabolic measurements, conducted at CRC, were carried
out on eight males during July and August 1983, on four
females and three males from November 1983 through March
1984, and on four females during June and July 1984.
Raccoons were housed throughout the study such that they
were constantly exposed to a natural cycle of temperature and
photoperiod. Weather records for the Front Royal area indicate
that average temperatures are around -0.5°C in January and
23.3°C in July (Crockett, 1972). Light:dark (L:D) periods for
the latitude of CRC (48°55'N; United States Department of the
Interior Geological Survey, 1972), calculated from duration of
daylight tables (List, 1971:506-512), were 14.9:9.1 and
9.4:14.6 hours L:D for summer and winter solstices,
respectively, and 12.2:11.8 hours L:D for vernal and autumnal
equinoxes.
Our animals were fed a measured amount of food daily, and
they usually ate most of what was provided. Occasionally these
animals would eat very little or none of their ration, and on
some days they would eat all that was given to them. We fed
them either feline diet (ground horse meat) or canned mackerel
(Star-kist®[1]) along with high-protein dog chow (Purina®).
When available, fresh fruit also was added to their diet. Water
was always provided ad libitum.
[1] The use of product brand names in this publication is not intended as an endorsement of the products by the Smithsonian Institution.
Measurements were conducted during the raccoons' daily
inactive period (sunrise to sunset) in both summer and winter.
Oxygen consumption was measured in a flow-through metabolism
chamber at 5°C intervals from -10°C to 35°C. Animals
were held at each temperature until the lowest rate of oxygen
consumption had been obtained and maintained for at least 15
minutes. During each determination, oxygen consumption was
monitored for 30 minutes to one hour beyond a suspected
minimum value to see if an even lower reading could be
obtained. Raccoons attained minimum levels of oxygen
consumption more quickly at warm (>10°C) than at cold
[Pg 7]
temperatures. Depending on the temperature, therefore, each
measurement took from two to five hours to complete. On days
when two measurements could be completed, the second trial
was always at a temperature 10°C warmer than the first.
The metabolism chamber was constructed from galvanized
sheet metal (77.5 × 45.5 × 51.0 cm = 180 liters) and was painted
black inside. Within the chamber, the animal was held in a cage
(71 × 39 × 33 cm) constructed from turkey wire that also was
painted black. This cage prevented the raccoons from coming
into contact with the walls of the chamber, yet it was large
enough to allow them to stand and freely move about. The
bottom of the cage was 11 cm above the chamber floor, which
was covered to a depth of one cm with mineral oil to trap urine
and feces.
During measurements, the metabolism chamber was placed
in a controlled-temperature cabinet (modified Montgomery
Ward model 8969 freezer). Air temperature (Ta) in the
metabolism chamber was regulated with a Yellow Springs
Instrument model 74 temperature controller. Ta was controlled
to ± 1.0°C at temperatures below freezing, and to ± 0.5°C at
temperatures above freezing. The chamber air and wall
temperatures were recorded continuously (Linseis model
LS-64 recorder) during each experiment, and, except during
temperature changes, they were always within 0.5°C of each
other.
Columns of Drierite® and Ascarite® removed water vapor
and carbon dioxide, respectively, from air entering and leaving
the chamber. Dry carbon-dioxide-free room air was pumped
into the chamber (Gilman model 13152 pressure/vacuum
pump) at a rate of 3.0 L/min (Gilmont model K3203-20 flow
meter). Downstream from the chemical absorbents, an aliquot
(0.1 L/min) of dry carbon-dioxide-free air was drawn off the
chamber exhaust line and analyzed for oxygen content
(Applied Electrochemistry model S-3A oxygen analyzer,
model 22M analysis cell, and model R-1 flow control). All gas
values were corrected to standard temperature and pressure for
dry gas. Oxygen consumption was calculated from the
difference in oxygen content between inlet and outlet air using
Eq. 8 of Depocas and Hart (1957).
Each raccoon was fasted for at least 12 hours before oxygen
consumption measurements began. At the start and end of each
metabolic trial the animal was weighed to the nearest 10 g
(Doctors Infant Scale, Detecto Scales, Inc., Brooklyn, N.Y.,
U.S.A.). The body mass used in calculating minimum oxygen
consumption and evaporative water loss was estimated from
timed extrapolations of the difference between starting and
ending weights, and the time at which these variables were
measured.
During metabolic measurements at temperatures above
freezing, evaporative water loss was determined gravimetrically.
Upstream from the chemical columns, an aliquot of air
(0.1 L/min) was drawn off the exhaust line and diverted for a
timed interval through a series of preweighed (0.1 mg) ![]() -tubes
-tubes
containing Drierite®. The aliquot then passed through a second
series of ![]() -tubes containing Ascarite® before entering the
-tubes containing Ascarite® before entering the
oxygen analysis system. Evaporative water loss was calculated
using Eq. 1
| Ė = (mw·.Ve)/(.Va·t·m) | Eq. 1 |
where Ė is evaporative water loss (mg·g-1·h-1), mw is mass of
water collected (mg), .Ve is rate of air flow into the chamber (3.0
L/min), .Va is the rate of air flow through the ![]() -tubes (0.1 L/min),
-tubes (0.1 L/min),
t is length of the timed interval (h), and m is the estimated mass
of the raccoon at the time of sampling (g).
Veterinarians at the National Zoological Park surgically
implanted calibrated temperature-sensitive radio transmitters
(Telonics, Inc., Mesa, AZ, U.S.A.) into abdominal cavities of
two female and two male raccoons. Transmitter pulse periods
were monitored with a digital processor (Telonics TDP-2)
coupled to a receiver (Telonics TR-2-164/166). During some
metabolic measurements, body temperatures of these animals
were recorded to the nearest 0.1°C at 30-minute intervals. The
daily cycle of body temperature of these raccoons also was
measured once a month.
At the conclusion of these experiments, the accuracy of our
calorimetry apparatus was tested by burning an ethanol lamp in
the metabolism chamber. During these tests a CO2 analyzer was
incorporated into the system (Beckman, LB-2). Results
demonstrated that we measured 84% of the oxygen consumed
by the lamp as well as 84% of the water and CO2 it produced;
standard deviation = ± 2.6, ± 5.0, and ± 3.6, respectively (n =
27). Average respiratory quotient (RQ) calculated from these
data was O.657 ± 0.008 (n = 27), which is 99.5% of that
predicted (0.66). McNab (1988b) reports that the accuracy of
open-flow indirect calorimetry systems, such as ours, depends
on the rate of air flow through the animal chamber. If flow rates
are too low, there is inadequate mixing of air within the
chamber, and the rate of oxygen consumption, as calculated
from the difference in oxygen content of air flowing into and
out of the chamber (Depocas and Hart, 1957), is underestimated.
At some critical rate of air flow, which is unique to each
combination of chamber and animal, this situation changes
such that measured rates of oxygen consumption become
independent of any further increase in flow rate (McNab,
1988b). In recent tests of our system, where we burned the
ethanol lamp at a variety of chamber flow rates, the efficiency
[Pg 8]
of measurement increased linearly as flow rate increased, and
the critical rate of air flow was about 6.7 L/min. This appeared
to explain why a flow rate of 3.0 L/min underestimated oxygen
consumption of the ethanol lamp.
Our earlier tests of the efficiency of our system indicated that
although we underestimated actual oxygen consumption of the
ethanol lamp, we did so with a fair degree of precision;
probably because flow rates were closely controlled. During
our metabolic measurements, chamber flow rates also were
closely controlled at 3.0 L/min, and we believe, therefore, that
these measurements also were carried out with a high degree of
precision. Consequently, all measured values of oxygen
consumption and water production were considered to be 84%
of their actual value and were adjusted to 100% before being
included in this report.
The calibration of all temperature-sensitive radio transmitters
drifted over time. Transmitters were calibrated before they
were surgically implanted and again after they were removed
from the animals. Although the drift of each transmitter was
unique, it was also linear (S. Tomkiewicz, Telonics, Inc., pers.
com.). All body temperature measurements were corrected
from timed extrapolations of the difference between starting
and ending calibrations.
Values of oxygen consumption, evaporative water loss, and
body temperature were plotted as a function of chamber air
temperature. Linear regressions of oxygen consumption at
temperatures below the thermoneutral zone (Tn), and evaporative
water loss at temperatures above freezing, were determined
with the SAS (1982) GLM procedure. Lower critical temperature
(Tlc) was determined graphically from intersection of the
line representing Ḣb and the regression line representing
oxygen consumption below Tn. Slopes and intercepts of
regression lines, as well as other mean values, were compared
with t-tests (Statistical Analysis System, 1982; Ott, 1984:138-175).
Unless indicated otherwise, data are expressed as mean
± standard deviation (s.d.).
We employed the method first described by Cole (1954) to
calculate rmax:
| 1 = e-rmax + b·e-rmax(a) - b·e-rmax(n+1) | Eq. 2 |
where a is potential age of females first producing young, b is
potential annual birth rate of female young, and n is potential
age of females producing their final young. After life-history
data were substituted into Eq. 2, rmax was determined by trial
and error substitution (Hennemann, 1983).
Because rmax represents the genetically fixed, physiologically
determined maximum possible rate of increase, data on
earliest possible age of female reproduction, highest possible
birth rate of female young, and longest possible female
reproductive life span were used for a, b, and n, respectively.
Calculated values, therefore, represent physiologically possible,
not ecologically possible, intrinsic rates of increase
(Hennemann, 1983, 1984; Hayssen, 1984; McNab, 1984b).
Values of n were derived from longevity records for captive
animals, and as these were all large values of similar duration
(14-16 years), they had very little effect on rmax. All species
considered have one litter per year, and because their sex ratios
at birth are about 50:50, variation in b was due to differences
in litter size. Therefore, age of first reproduction and litter size
had the greatest effect on rmax. Intrinsic rate of increase scales
to body mass (Fenchel, 1974), and we removed this effect by
comparing each calculated rmax with the value expected (rmaxe)
on the basis of body mass (Hennemann, 1983).
Dimensionless numbers for each of the four variables used in
calculating composite scores were derived as follows. Ratios of
measured to predicted values were used for basal metabolism
(Hbr) and minimum wet thermal conductance (Cmwr). Thermoregulatory
ability at low temperatures is closely related to
the ratio Hbr/Cmwr (McNab, 1966). This ratio was used,
therefore, to gauge each species' cold tolerance. For Dd we used
the ratio of food categories actually used by a species to the
total number of food categories taken by all species tested (Ddr).
The ratio of calculated to expected intrinsic rates of natural
increase was used to derive rmaxr. Composite scores were
calculated as
| Composite score = [(Hbr/Cmwr) + Ddr + rmaxr]/3 | Eq. 3 |
The correlation between number of climates these species
occupy and their composite scores was tested by linear
regression.
According to monthly live-trapping records, the body mass
of free-ranging female raccoons increased from 3.6 ± 0.6 kg
during summer to 5.6 ± 0.8 kg in early winter, and the mass of
free-ranging males increased from 4.0 ± 0.5 to 6.7 ± 0.9 kg
during the same interval. These seasonal changes in body mass
were due to fluctuations in the amount of body fat and represent
a mechanism for storing energy during fall for use in winter. In
summer, captive and trapped male and captive female raccoons
had the same body mass (4.73 ± 0.61, 4.41 ± 0.70, and 4.67
[Pg 9]
± 0.88 kg, respectively, Table 2). Mass of captive females did
not change between seasons, whereas captive males were
heavier in winter than summer (p<0.005; Table 2). This
seasonal change in mass of our captive males was of a much
smaller magnitude (0.6 kg) than that observed for wild males
(2.7 kg). During winter, captive males (5.34 ± 1.39 kg) were
heavier than captive females (4.49 ± 0.98 kg; p<0.005; Table
2). Thus, our captive animals maintained a body mass
throughout the year that was intermediate to the range of values
found for wild raccoons in the same area.
winter (s.d. = standard deviation and n = number of observations).
| Season and sex | Body mass, ± s.d., | (n) | Basal metabolism, ± s.d., | (n) |
|---|---|---|---|---|
| Summer | ||||
| Trapped male | 4.41 ± 0.70 | (52) | 780 ± 112 | (20) |
| Captive male | 4.73 ± 0.61 | (22) | 680 ± 102 | (8) |
| Captive female | 4.67 ± 0.88 | (41) | 618 ± 92 | (13) |
| Winter | ||||
| Captive male | 5.34 ± 1.39 | (31) | 704 ± 81 | (19) |
| Captive female | 4.49 ± 0.98 | (42) | 667 ± 139 | (25) |
Within thermoneutrality, Ḣb (mL O2·g-1·h-1) was 0.54 ± 0.09
for trapped males in summer, 0.46 ± 0.07 for captive males in
summer, 0.42 ± 0.07 for captive females in summer, 0.47 ± 0.06
for captive males in winter, and 0.46 ± 0.10 for captive females
in winter (Figures 2, 3). Ratios of these measured values to
those predicted by the Kleiber (1932, 1961:206) equation are
1.28, 1.12, 1.02, 1.17, and 1.09, respectively. To minimize the
effect of body size (Mellen, 1963) and to facilitate comparisons
between sexes and seasons and between captive and trapped
animals, basal metabolism also was calculated as a function of
metabolic body size (mL O2·kg-0.75·h-1; Table 2). Based on this
analysis, trapped summer males had a higher basal metabolism
than captive males (p<0.025) or females (p<0.005) in either
season (Table 2). There was no difference in basal metabolism
between captive males and females in either summer or winter,
and there was no seasonal difference in their basal metabolic
rates (Table 2).
[↑ TOC]
Minimum wet and dry thermal conductances were calculated
using Eqs. 4 and 5
| Cmw = Ḣr / (Tb - Ta) | Eq. 4 |
| Cmd = (Ḣr - Ėeq) / (Tb - Ta) | Eq. 5 |
where Cmw is wet and Cmd is dry conductance (mL
O2·g-1·h-1·°C-1); Ḣr is the lowest resting metabolic rate
measured at each temperature (mL O2·g-1·h-1);
Ėeq is oxygen equivalent for heat lost by evaporation
[Ėeq = mL O2·g-1·h-1 = Ė·λ/γ, where Ė is evaporative water loss
(mg·g-1·h-1), λ is heat of vaporization for water (2.43 J/mg), and
γ is heat equivalent for oxygen (20.097 J/mL)]; Tb is body
temperature (°C); and Ta is chamber air temperature (°C). Only
data from animals equipped with temperature-sensitive radio
transmitters were used for these calculations.
winter. Means of values were calculated from equations 3 and 4 (s.d. = standard deviation and n = number of
observations).
| Season and sex | Thermal conductance | ||||
|---|---|---|---|---|---|
| Wet ± s.d. | (n) | Dry ± s.d. | (n) | ||
| Summer | |||||
| Captive, both sexes | 0.0256 ± 0.0028 | (18) | 0.0246 ± 0.0019 | (12) | |
| Winter | |||||
| Captive, female | 0.0172 ± 0.0023 | (10) | 0.0161 ± 0.0027 | (6) | |
[Pg 10]
[Pg 11]
Cmw was calculated for each season from metabolic
measurements made at all air temperatures below Tlc (Table 3).
Because evaporative water loss was not measured at temperatures
below freezing, Cmd was calculated only from metabolic
determinations made at air temperatures between Tlc and 0°C.
There was no difference between males and females in summer
for either Cmw or Cmd (mL O2·g-1·h-1·°C-1). Data for each sex
were combined to give a summer average of 0.0256 ± 0.0028
for Cmw, and 0.0246 ± 0.0019 for Cmd (Table 3). These summer
conductances were 49% higher (p<0.005) than those calculated
for winter females (0.0172 ± 0.0023, and 0.0161 ± 0.0027 for
Cmw and Cmd, respectively; Table 3). Cmw and Cmd were not
different from each other in either summer or winter, which
indicated that in both seasons evaporative water loss contributed
very little to heat dissipation at temperatures below Tn.
Comparisons of thermal conductances calculated on the basis
of metabolic body size (Mellen, 1963) gave the same results.
Evaporative water loss increased as chamber temperature
increased in both summer and winter (Figures 4, 5). In summer,
the pattern of increase was different for females and males.
Polynomial regressions for trapped and captive males produced
equations that describe a concave relationship between Ta and
evaporative water loss, whereas the equation for females
describes a sigmoid curve (Table 4; Figure 4). For females,
water loss increased rapidly at temperatures above 25°C
(Figure 4). The intercepts and coefficients of the X, X2, and X3
terms of the polynomial regression equations (Table 4) were
compared (t-tests) to determine if they differed from each other.
The coefficients in the equation for trapped males differed from
those for captive females in the X2 (p<0.05) and X3 (p<0.025)
terms. The intercept and coefficients of the equation for captive
males, however, were not different from those for either captive
females or trapped males. Although this lack of difference is
understandable in the case of trapped males, where the shape of
the two curves is similar (concave), it is not so clear for the
sigmoid curve of captive females (Figure 4). Perhaps the lack
of difference in this case is simply due to the small number of
observations available for captive males (n = 10; Table 4).
Nonetheless, in summer at 35°C, both captive and trapped
males relied less on evaporative cooling than did captive
females (Figure 4).
In winter, males and females had similar rates of evaporative
water loss across the full range of temperatures tested (Figure
5). Therefore, data for both sexes were combined. The intercept
and coefficients of this equation (Table 4) did not differ from
those for summer females, but they did differ from those in the
regression for trapped males in the X2 (p<0.05) and X3
(p<0.025) terms. As was the case for females in summer,
rates of water loss for winter animals increased most rapidly at
temperatures above 25°C (Figure 5).
captive females, open circles; captive males, closed circles; trapped males, open squares. Lines represent
polynomial regressions of evaporative water loss on chamber air temperature.
summer and winter (X = chamber temperature (°C), Y = evaporative water loss, n = number of observations, R2
= coefficient of determination, and SEE = standard error of estimate).
| Season and sex | Equation | (n) | R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Summer | ||||||||||
| Trapped male | Y = | 0.1899 | + | 0.0114 X | + | 0.0011 X2 | - | 0.00002 X3 | (32) | 0.86 |
| SEE | 0.0885 | 0.0223 | 0.0015 | 0.00003 | ||||||
| Captive male | Y = | 0.2174 | + | 0.0192·X | + | 0.0009·X2 | - | 0.00003·X3 | (10) | 0.73 |
| SEE | 0.3983 | 0.0834 | 0.0048 | 0.00008 | ||||||
| Captive female | Y = | 0.0127 | + | 0.0943·X | - | 0.0060·X2 | + | 0.00013·X3 | (31) | 0.64 |
| SEE | 0.2218 | 0.0547 | 0.0036 | 0.00006 | ||||||
| Winter | ||||||||||
| Captive, both sexes | Y = | 0.1550 | + | 0.0426·X | - | 0.0025·X2 | + | 0.00006·X3 | (57) | 0.80 |
| SEE | 0.0734 | 0.0192 | 0.0013 | 0.00002 | ||||||
Body temperatures in Figure 6 are those recorded during
metabolic measurements from animals equipped with surgically
implanted, temperature-sensitive radio transmitters. Each
point was recorded during the lowest level of oxygen
consumption at each Ta. In both summer and winter, Tb's were
lowest during metabolic measurements at Ta's around Tlc. At
Ta's below Tlc, Tb's increased (Figure 6), which is an unusual
[Pg 13]
response. Under similar conditions, other procyonids either
maintain a nearly constant Tb or allow it to fall slightly (Müller
and Kulzer, 1977; Chevillard-Hugot et al., 1980; Müller and
Rost, 1983; Chevalier, 1985). For our raccoons, confinement in
the metabolism chamber at low temperatures must have
stimulated a greater than necessary increase in metabolic rate
such that heat production exceeded heat loss, which caused Tb
to become elevated.
their lower critical temperature (I = x-intercept (°C), n = number of observations, R2 = coefficient of determination, SEE
= standard error of estimate for the y-intercept (a) and slope (b), X = chamber temperature (°C), and Y = oxygen consumption).
| Season and sex | Equation | (n) | R2 | SEE | I | |
|---|---|---|---|---|---|---|
| a | b | |||||
| Summer | ||||||
| Trapped male | Y = 1.09 - 0.0281·X | (30) | 0.64 | 0.0353 | 0.0040 | 38.8 |
| Captive male | Y = 0.97 - 0.0258·X | (12) | 0.91 | 0.0235 | 0.0025 | 37.6 |
| Captive female | Y = 1.04 - 0.0251·X | (29) | 0.78 | 0.0288 | 0.0026 | 41.1 |
| Winter | ||||||
| Captive, both sexes | Y = 0.68 - 0.0193·X | (36) | 0.68 | 0.0157 | 0.0023 | 35.2 |
During summer, Tlc for male raccoons was 20°C, whereas for
females it was 25°C (Figure 2). Regression equations calculated
to describe oxygen consumption at Ta's below Tlc are
presented in Table 5. For three groups of summer animals,
slopes of regressions are identical. This indicates that minimum
conductances of these three groups were equivalent. Intercepts
of these equations are different, which suggests a difference in
metabolic cost of thermoregulation between these groups
(Figure 2); captive males had a lower intercept than either
trapped males (p<0.005) or captive females (p<0.05), but there
was no difference in intercepts of captive females and trapped
males. These regression equations, therefore, also were derived
using values of oxygen consumption expressed in terms of
metabolic body mass (Mellen, 1963). Relationships between
intercepts of these equations are different than those for
regressions in Table 5. Intercept for females was intermediate
to, and not different from, those of the two groups of males.
However, captive males still had a lower intercept than trapped
males (p<0.025). Thus, in summer, thermoregulatory metabolism
was less expensive for captive than for trapped males, and
in spite of a 5°C difference in their Tlc's (Figure 2), captive
males and females had similar thermoregulatory costs.
Regression lines for three groups of animals in summer
extrapolate to zero metabolism at values equivalent to, or
greater than, normal Tb; 38.8°C for trapped males, 37.6°C for
captive males, and 41.1°C for captive females (Table 5). Thus,
all three groups had minimized thermal conductance at Ta's
below Tlc (Scholander et al., 1950b; McNab, 1980b). Minimum
wet thermal conductance calculated for raccoons in summer
with Eq. 4 (Table 3) is numerically similar to these "slope"
values (Table 5), and it was, therefore, considered to be the best
estimate of Cmw for Procyon lotor during that season (0.0256
mL O2·g-1·h-1·°C-1).
During winter Tlc for both sexes decreased to 11°C (Figure 3).
Regression equations of thermoregulatory metabolism for
males and females in winter are not different from each other in
either slope or intercept. These data, therefore, were combined
into a single equation (Table 5). Slope and intercept of this
equation are both lower (p<0.005 and p<0.05, respectively)
than those for summer animals (Table 5). Identical results were
obtained from comparisons using regressions derived from
oxygen consumption expressed in terms of metabolic body
mass (Mellen, 1963). Thermoregulatory costs at any temperature
below 20°C were lower for winter than summer animals
(Figures 2, 3).
O2·g-1·h-1) of Procyon lotor at temperatures below their lower critical
temperature in winter (A = females with radio transmitters, B = females without
radio transmitters, C = males, I = x-intercept (°C), n = number of observations,
R2 = coefficient of determination, X = chamber temperature (°C), and Y =
oxygen consumption).
| Group | Equation | (n) | R2 | I |
|---|---|---|---|---|
| A | Y = 0.63 - 0.0158·X | (10) | 0.66 | 40.1 |
| B | Y = 0.72 - 0.0226·X | (11) | 0.71 | 32.1 |
| C | Y = 0.69 - 0.0200·X | (15) | 0.79 | 34.7 |
The regression line for Procyon lotor in winter (Table 5)
extrapolates to zero metabolism at 35.2°C, which is below
normal Tb (Figures 6, 7). This suggests that not all raccoons
measured in winter minimized thermoregulatory metabolism or
conductances at Ta's below Tlc (Scholander et al., 1950b;
McNab, 1980b). To assess this possibility, data for these
animals were divided into three groups: (A) females with radio
transmitters, (B) females without radio transmitters, and (C)
males (Table 6). Regression equations of metabolism below Tlc
were derived for each group, and based on extrapolated Tb's at
zero metabolism, only the two females with implanted radio
transmitters (group A) minimized thermoregulatory metabolism
and conductance. Had animals in groups B and C also
minimized their thermal conductances, while retaining their
measured metabolic rates, their rates of heat production would
have been disproportionately higher than their rates of heat
loss. Equation 4 predicts that under these conditions their body
temperatures would have been elevated to 42.0°C and 40.4°C,
respectively. Thus, in order to avoid such a large increase in
body temperature, animals in groups B and C increased their
thermal conductances in preference to lowering their metabolic
[Pg 16]
rates. The regression equation of thermoregulatory metabolism
for all winter animals (Table 5), therefore, overestimates
minimum metabolic cost of temperature regulation below Tlc,
and its slope underestimates Cmw. Consequently, the best
estimate of Cmw for Procyon lotor in winter is the value
calculated for group A animals with Eq. 4 (0.0172 mL O2·g-1·h-1·°C-1;
Table 3), and the minimum cost of thermoregulatory
metabolism at any Ta below Tlc is best estimated by
substituting this value into Eq. 4 and solving for Ḣr.
In both summer and winter, Tb's increased during metabolic
measurements at Ta's above Tlc (Figure 6). This response also
was seen during metabolic measurements conducted on other
procyonids (Müller and Kulzer, 1977; Chevillard-Hugot et al.,
1980; Müller and Rost, 1983; Chevalier, 1985).
During summer our data suggested that the upper critical
temperature (Tuc) was higher than 35°C. The lowest rates of
oxygen consumption at Ta = 35°C occurred after 1.5 to 2.5
hours of exposure to that temperature. Prolonged exposure to
this temperature in summer did not make animals restless, and
their rate of oxygen consumption was very stable throughout
each measurement. Body temperature responses at Ta = 35°C
were recorded from two males and two females that had
implanted radio transmitters. With the exception of one male,
Tb's were maintained near 38°C (Figure 6). The one exception
(a male) maintained its Tb at 39.3°C. At Ta = 35°C, summer
males had rates of evaporative water loss that were lower than
those of summer females (Figure 4). At this temperature, males
dissipated 35% ± 6% and females 56% ± 18% of their metabolic
heat via evaporative water loss. Thus, at Ta = 35°C, males must
have utilized modes of heat transfer other than evaporative
cooling (convective and conductive heat transfer) to a greater
extent than females.
Body temperature, evaporative water loss, and metabolic
data indicated that, in winter, Tuc was very close to 35°C. In
winter, the lowest level of oxygen consumption was recorded
during the first hour after the chamber had reached Ta = 35°C.
Unlike summer, animals became restless after the first hour at
35°C, at which point their oxygen consumption increased and
showed a high degree of variability. Body temperature
responses at 35°C were recorded from both females that had
implanted radio transmitters. In one case, Tb rose from 37.9°C
at the end of the first hour to 40.5°C by the end of the second
hour, and as it did not show signs of leveling off, we terminated
the experiment. We exposed that same animal to Ta = 35°C one
other time during winter. In that instance, its Tb rose to 40.0°C
during the first 30 minutes and was maintained at that level for
three hours with no apparent distress. The other female elevated
its Tb from 37.3°C to 39.0°C during the second hour at
Ta = 35°C and maintained its Tb at that level for
two hours. Thus, during winter, prolonged exposure to
Ta = 35°C stimulated more of an increase in Tb than it did in
summer. During winter, both males and females increased
evaporative water loss at Ta = 35°C (Figure 5) but only to the
extent that they dissipated 35% ± 10% of their metabolic heat
production. Thus, even in winter, convective and conductive
heat transfers were still the most important modes of heat loss
at this temperature.
The daily cycle of raccoon Tb's during summer and winter
are presented in Figure 7. In general, Tb's showed a marked
circadian cycle in phase with photoperiod. Tb's rose above
38°C for several hours each night but remained below 38°C
during daytime. During summer, with the exception of one
female whose record was not typical (Figure 7), Tb's rose above
38°C shortly after sunset, whereas in winter Tb's did not rise
above 38°C until several hours after sunset. Once Tb was
elevated it usually remained so until just before or after sunrise
(Figure 7). During summer, Tb was above 38°C for 85% or
more of the time between sunset and sunrise (87% for the
female with the typical body temperature pattern, and 85% and
98% for males), whereas in winter it was elevated for only
47%-78% of the time between sunset and sunrise (47% and
61% for females, and 67% and 78% for males). During night,
Tb would oscillate between 38°C and about 39°C, such that two
peak values occurred. These peak values presumably corresponded
to two periods of heightened nighttime activity.
During summer, one of these peaks occurred before and the
other after 24:00 hours, whereas in winter both peaks occurred
after 24:00 hours. With the exception of one female in winter
(Figure 7), the lowest Tb of the day for both sexes was near
37°C, and this typically occurred during daytime (Figure 7).
Basal metabolism represents the minimum energy required
by a mammal to maintain endothermy and basic homeostasis
(Lusk, 1917:141; Kleiber, 1932, 1961:251; Benedict, 1938:191-215;
Brody, 1945:59; Robbins, 1983:105-111). Mammals
with lower than predicted Ḣb maintain endothermy and
enjoy its attendant advantages at a discount, whereas others,
with rates that are higher than predicted, pay a premium
[Pg 17]
(Calder, 1987). Such variation in Ḣb appears to be tied to
ecological circumstances rather than taxonomic affinities
(Vogel, 1980; McNab, 1986a, 1988a, 1989), and depending on
environmental conditions, each rate provides an individual
with various advantages and limitations. During the course of
evolution, therefore, each species' Ḣb evolves to provide it with
the best match between its energy requirements for continuous
endothermy, its food supply, and the thermal characteristics of
its environment.
Male raccoons trapped in summer had higher Ḣb's than our
captive animals in any season (Table 2). The higher rate of
metabolism of these trapped males could have been due to the
stress of captivity or to the fact that "wild" animals actually
may have higher metabolic rates than those that have adjusted
to captivity. If the latter is true, then our data for captive
animals underestimated the actual energy cost of maintenance
metabolism for Procyon lotor in the wild. At present, we have
no way of determining which of these alternatives is true.
In some temperate-zone mammals, Ḣb is elevated in winter,
which presumably increases their "cold-hardiness." Conversely,
lower summer metabolism is considered to be a
mechanism that reduces the potential for heat stress. Such
seasonal variation in Ḣb has been found in several species:
collard peccary, Tayassu tajacu (Zervanos, 1975); antelope
jackrabbit, Lepus alleni (Hinds, 1977); desert cottontail,
Sylvilagus audubonii (Hinds, 1973); and, perhaps, cold-acclimatized
rat, Rattus norvegicus (Hart and Heroux, 1963).
Unlike these species, our captive raccoons showed no seasonal
variation in Ḣb (Table 2). Instead, raccoons achieved "cold-hardiness"
in winter and reduced their potential for heat stress
in summer with a large seasonal change in thermal conductance
(Table 3).
Table 7.—Metabolic characteristics of several procyonid species.
| Species | Body Mass (g) | Basal[a] metabolism | Minimum[b] conductance | Tb[c] | Tn[d] | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meas | Hbr | Meas | Cmwr | α | ρ | Tlc | Tuc | ||||||||
| Bassariscus astutus | 865 | 0.43 | 0.68 | 0.0288[e] | 0.85 | 37.6 | 23 | 35.5 | Chevalier (1985) | ||||||
| Procyon cancrivorus | 1160 | 0.40 | 0.69 | 0.0368[e] | 1.25 | 26 | Scholander et al. (1950b, c) | ||||||||
| Potos flavus | 2030 | 0.36 | 0.51 | McNab (1978a) | |||||||||||
| Potos flavus | 2400 | 0.32 | 0.65 | 38.1 | 36.0 | 23 | 30 | Müller and Kulzer (1977) | |||||||
| Potos flavus | 2600 | 0.34 | 0.71 | 0.0200[f] | 1.02 | 23 | 33 | Müller and Rost (1983) | |||||||
| Nasua nasua | 3850 | 0.26 | 0.60 | 0.0200[f] | 1.24 | 38.3 | 36.4 | 25 | 33 | Chevillard-Hugot et al. (1980) | |||||
| Nasua nasua | 4847 | 0.33 | 0.79 | 0.0238[e] | 1.65 | 39.1 | 37.9 | 30 | 35 | Mugaas et al. (in prep.) | |||||
| Nasua narica | 5554 | 0.25 | 0.62 | 0.0208[e] | 1.55 | 38.9 | 37.4 | 25 | 35 | ||||||
| Nasua narica | 4150 | 0.42 | 1.20 | 0.0341[e] | 2.20 | Scholander et al. (1950b, c) | |||||||||
| 0.0224[g] | 1.45 | ||||||||||||||
| Procyon lotor | This study | ||||||||||||||
| Summer | |||||||||||||||
| Trapped male | 4400 | 0.54 | 1.28 | 20 | |||||||||||
| Captive male | 4790 | 0.46 | 1.07 | 0.0256[f] | 1.77 | 38.4 | 37.5 | 20 | |||||||
| Captive female | 4670 | 0.42 | 1.02 | 0.0256[f] | 1.79 | 38.2 | 37.6 | 25 | |||||||
| Winter | |||||||||||||||
| Captive male | 5340 | 0.47 | 1.17 | 38.6 | 38.6 | 11 | |||||||||
| Captive female | 4490 | 0.46 | 1.10 | 0.0172[f] | 1.15 | 38.3 | 37.3 | 11 | |||||||
[a]
Meas is measured basal metabolism (mL O2·g-1·h-1). Hbr is the ratio of measured to predicted basal metabolism where the predicted value is calculated from Ḣb
= 3.42·m-.25 (Kleiber, 1932, 1961:206) and m is body mass in grams.
[b] Meas is measured minimum thermal conductance (mL O2·g-1·h-1·°C-1). Cmwr is the ratio of measured to predicted minimum thermal conductance where the predicted value is calculated from Cm = 1.0·m-0.5 (McNab and Morrison, 1963; Herreid and Kessel, 1967), and m is body mass in grams.
[c] Tb is body temperature during the active (α) and rest (ρ) phases of the daily cycle (°C).
[d] Tn is the thermoneutral zone as defined by the lower (Tlc) and upper (Tuc) critical temperatures (°C).
[e] Conductance calculated as the slope of the line describing oxygen consumption at temperatures below the lower critical temperature.
[f] Conductance calculated from Cmw = Ḣr/(Tb - Ta), where Ḣr is resting metabolic rate at temperatures below Tlc, and other symbols are as described elsewhere.
[g] Inactive-phase thermal conductance: estimated from Scholander et al. (1950b), assuming that active-phase thermal conductance is 52% higher than values determined during the inactive phase (Aschoff, 1981).
Procyon lotor has a much higher mass-specific Ḣb than other
procyonids (Table 7). To quantify the magnitude of this
difference, we compared the measured value for Procyon lotor
[Pg 18]
with one calculated for it from a mass-specific least-squares
regression equation (Eq. 6; R2 = 0.78) derived from data for
those procyonids with lower than predicted Ḣb: Potos flavus,
Procyon cancrivorus, Nasua nasua, Nasua narica, and
Bassariscus astutus (Table 7).
| Ḣb = 2.39·m-0.25 | Eq. 6 |
Ḣb in Eq. 6 is basal metabolism (mL O2·g-1·h-1) and m is body
mass (g). Measured values of Ḣb for Procyon lotor were 1.45 to
1.86 times greater than those predicted for it by Eq. 6 (Table 8).
Eq. 6 (Ḣb = 2.39·m-0.25). Body masses, used to calculate predicted values, and
measured values were taken from Table 7.
| Season and sex | Predicted | Measured/Predicted | |
|---|---|---|---|
| Summer | |||
| Trapped male | 0.29 | 1.86 | |
| Captive male | 0.29 | 1.59 | |
| Captive female | 0.29 | 1.45 | |
| Winter | |||
| Captive male | 0.28 | 1.68 | |
| Captive female | 0.29 | 1.59 | |
Background.—With respect to Ḣb, McNab (1986a:1)
maintains that "the influence of climate is confounded with the
influence of food habits," and that departures from the Kleiber
(1961) "norm" are best correlated with diet. Although this does
appear to be the case for diet specialists, the analysis is not so
clear-cut for omnivorous species (McNab, 1986a). His analysis
also indicates that an animal's "behavior" (i.e., whether it is
terrestrial, arboreal, subterranean, aquatic, etc.), secondarily
modifies the influence of food habits on Ḣb. For example,
terrestrial frugivores have Ḣb's that are very near predicted
values, whereas arboreal frugivores have rates that are much
lower than predicted (McNab, 1986a).
| + | <20% by volume when found. | † | 1%-19% frequency of occurrence. |
| ++ | >20% by volume when found. | †† | 20%-50% frequency of occurrence. |
| ††† | >50% frequency of occurrence. | ||
| Food | Potos flavus | Procyon cancrivorus | Nasua nasua | Nasua narica | Bassariscus | Procyon lotor | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mammalia | + | † | # | ++ | ††† | ++ | †† | |||||
| Aves | ++ | † | + | †† | ||||||||
| Birds' eggs | ††† | |||||||||||
| Reptilia | + | † | + | ††† | # | + | † | + | † | |||
| Amphibia | + | † | + | † | ||||||||
| Pices | ++ | †† | ++ | †† | ||||||||
| Insecta | ++ | † | + | ††† | ++ | ††† | # | + | †† | ++ | †† | |
| Arachnida | ++ | ††† | # | + | † | + | † | |||||
| Chilopoda | ++ | ††† | ||||||||||
| Diplopoda | # | + | † | |||||||||
| Crustacea | ++ | ††† | # | ++ | ††† | |||||||
| Mollusca | + | †† | # | + | †† | |||||||
| Annelida | # | + | † | |||||||||
| Nuts | ++ | †† | ||||||||||
| Grains | ++ | †† | ||||||||||
| Buds | + | † | ||||||||||
| Fruit | ++ | ††† | ++ | # | †† | ++ | ††† | |||||
| Leaves | + | † | ||||||||||
| Grass | + | † | ||||||||||
Food Habits of Procyonids.—Food habits of six procyonids
for which metabolic data are available are presented in
Table 9. All six species clearly have mixed diets. Compared to
other species, Procyon lotor is highly catholic in its diet, taking
food from almost twice as many categories as Nasua narica,
three times as many as Procyon cancrivorus, Nasua nasua, and
Bassariscus astutus, and nine times as many as Potos flavus.
[Pg 19]
For those species for which food habit data are quantified, we
used Eisenberg's (1981:247-251) substrate/feeding matrix
method, where "substrate" is analogous to McNab's (1986a)
"behavior," to construct the following feeding categories that
are based on the major food groups utilized by each species
(Table 9).
1. Potos flavus: (1) arboreal/frugivore, insectivore.
2. Procyon cancrivorus: (1) semiaquatic/crustacivore, molluscivore, insectivore, piscivore, carnivore.
3. Nasua nasua: (1) terrestrial/insectivore, arachnidivore, carnivore, frugivore.
4. Bassariscus astutus: (1) terrestrial/carnivore, insectivore, frugivore.
5. Procyon lotor: (1) terrestrial/carnivore, granivore, frugivore, insectivore; and (2) semiaquatic/crustacivore, molluscivore, insectivore, piscivore, carnivore.
Food Habits and Basal Metabolism.—The most important
foods in the diet of Procyon lotor are vertebrates, nuts,
seeds, and fruits (Table 9). These are the same foods that are
eaten by those dietary specialists that have Ḣb's equivalent to,
or higher than, values predicted for them by the Kleiber
equation (McNab, 1986a). The most important foods in the
diets of Potos flavus, Procyon cancrivorus, and Nasua nasua
are invertebrates and fruit (Table 9), and these foods are eaten
by dietary specialists that have lower than predicted Ḣb's
(McNab, 1986a). Major foods in the diet of Bassariscus astutus
are terrestrial vertebrates, insects, and fruit (Table 9). Dietary
specialists that eat terrestrial vertebrates have higher than
predicted Ḣb's, whereas those that feed on insects have Ḣb's
that are lower than predicted (McNab, 1986a). Year-round
utilization of vertebrates by Bassariscus astutus suggests that it
also should have a metabolic rate that is equivalent to or higher
than predicted, rather than lower (McNab, 1986a). However,
perhaps year-round inclusion of insects in its diet (Martin et al.,
1951; Taylor, 1954; Wood, 1954; Toweill and Teer, 1977;
Trapp, 1978), plus water-and energy-conserving advantages of
a low metabolic rate, each exert a stronger selective influence
on Ḣb than do vertebrates in its diet.
Summary.—The basal metabolic rate of these procyonids
does appear to be influenced by diet. But, it is apparent from
this family's evolutionary history and tropical origins that
climate also has had a profound influence on its member's
metabolism. The history of the family and the data presented
here (Table 7) suggest that lower than predicted Ḣb is a feature
that evolved very early as the primary metabolic adjustment to
a tropical climate. From this perspective, it could be argued that
climate would have been the major selective force determining
Ḣb, whereas food habits would have had a secondary influence.
Background.—McNab (1980a) suggested that if food is
not restricted during an animal's reproductive period, the factor
that will limit growth and reproduction will be the rate at which
energy can be used in growth and development. Under these
conditions, an increase in Ḣb would actually increase rmax
because it would provide a higher rate of biosynthesis, a faster
growth rate, and a shorter generation time. Hennemann (1983)
tested McNab's (1980a) premise and found a significant
correlation between rmax and metabolic rate, independent of
body size, for 44 mammal species. A low correlation
coefficient for this relationship, however, indicated to him
(Hennemann, 1983) that factors such as (1) food supply, (2)
thermal characteristics of the environment, and (3) brain size
also contribute toward shaping a species' reproductive potential,
particularly when these factors strongly influence rates of
biosynthesis or growth or for some reason alter generation time.
Results of our estimates of rmax for procyonids are presented in
Table 10.
Procyon lotor.—This species had the highest Ḣb and Dd, and
also had the highest rmax (1.34; Table 10). Such a high rmax may
infer that this trait evolved under conditions where food and
temperature were not limiting to reproduction. Under these
conditions selection could have favored those reproductive
characteristics sensitive to a higher Ḣb (biosynthesis, growth,
and generation time; McNab, 1980a). Procyon lotor's high
reproductive potential is due to its early age of first female
reproduction and its large litter size, characteristics that may
reflect metabolically driven increases in both biosynthesis and
growth.
Bassariscus astutus.—This species has a low Ḣb but an rmax
that was 124% of expected (Table 10). This suggests that rmax
evolved under conditions where food and temperature were not
limiting to reproduction. Reduced litter size should restrict this
species' reproductive potential and may be a reflection of its
low Ḣb. The factor that is responsible for increasing its
reproductive potential, however, is its early age of first female
reproduction. Bassariscus astutus is the smallest of these
procyonids, and even though it has a low Ḣb, its small mass
may contribute to its ability to reach adult size and sexual
maturity in its first year. The high quality of its diet (a high
proportion of small vertebrates; Table 9) also may be a factor
that is permissive to early female reproduction. Thus, small
body size and diet may be factors that have allowed this species
to evolve a higher than expected reproductive potential in spite
of its low Ḣb.
Nasua narica.—This species is one of the largest procyonids
(Table 7), and it possesses characteristics that should limit its
reproductive potential: lower than predicted Ḣb (Table 7), a
relatively low-quality diet (Kaufmann, 1962:182-198; Table
9), and delayed time of first reproduction (Table 10). In spite of
this, Nasua narica has a higher than expected rmax (111% of
predicted; Table 10). The life history feature that enhances
Nasua narica's reproductive potential, and increases rmax
beyond expected, is its large litter size. In this species females
live in bands. Each year just before their young are born these
bands break up, and each female seeks out a den for herself and
[Pg 20]
her litter. Once the young are able to leave the den
(approximately five weeks), bands reform. In this situation,
females not only care for their own young but also for those of
other females in the band (Kaufmann, 1962:157-159, 1982,
1987; Russell, 1983). This social structure may contribute to
this species' ability to produce large litters and in this way
increase its reproductive potential.
| Species | Body mass (g) | a | b | n | rmax | rmaxe[a] | rmaxr[b] | References |
|---|---|---|---|---|---|---|---|---|
| Procyon lotor | 4940 | 0.83 | 2.25 | 16 | 1.34 | 0.53 | 2.52 | Dunn and Chapman (1983); Eisenberg (1981:489); Kaufmann (1987); Lotze and Anderson (1979); Nowak and Paradiso (1983:981); Sanderson(1987); Stains (1956:28-31); This study |
| Bassariscus astutus | 900 | 0.83 | 1.50 | 14 | 1.02 | 0.82 | 1.24 | Kaufmann (1982, 1987); Nowak and Paradiso(1983:979, 980); Poglayen-Neuwall and Poglayen-Neuwall (1980); Poglayen-Neuwall and Toweill (1988); Russell (1983) |
| Nasua narica | 3900 | 2.50 | 2.25 | 14 | 0.62 | 0.56 | 1.11 | Kaufmann (1982, 1987); Nowak and Paradiso (1983:983); Sanderson (1983) |
| Nasua nasua | 3850 | Chevillard-Hugot et al. (1980) | ||||||
| Procyon cancrivorus | 1160 | 0.83 | 1.50 | 15 | 1.02[c] | 0.77 | 1.32 | Crandall (1964:312); Poglayen-Neuwall (1987) |
| 1.75 | 0.65[c] | 0.84 | ||||||
| Potos flavus | 2490 | 1.75 | 0.50 | 12 | 0.30 | 0.63 | 0.48 | Ford and Hoffmann (1988); Nowak and Paradiso (1983:984) |
| Bassaricyon gabbii | 1600 | 1.75 | 0.50 | 15 | 0.32 | 0.71 | 0.45 | Eisenberg (1981:489); Nowak and Paradiso (1983:985) |
[a] rmaxe = 4.9·m0.2622, where m is body mass in grams.
[b] Regression of rmax on body mass (m). Assume rmax = 1.02 for Procyon cancrivorus: rmax = 0.00005·m + 0.623; R = 0.19; R2 = 0.03; Regression of rmaxr (Table 10) on Hbr (Table 7); assume Nasua nasua has the same rmaxr as Nasua narica: rmaxr = 3.35·Hbr - 1.11; R = 0.93; R2 = 0.86.
[c] Estimate based on females reproducing in their first (a = 0.83) or second (a = 1.75) year.
Nasua nasua.—Unfortunately, there is not enough reproductive
data to allow calculation of rmax for Nasua nasua (Table
10), therefore, it is not possible to compare the reproductive
potential of this South American coati with its North American
relative, Nasua narica. Given its low Ḣb and relatively
low-quality diet of fruit and terrestrial invertebrates (Table 9),
however, rmax of Nasua nasua may be very similar to that of
Nasua narica.
Procyon cancrivorus.—The age of first female reproduction
for Procyon cancrivorus has not been reported. However, if one
assumes females can reproduce in their first year, rmax for
Procyon cancrivorus would be 1.02 (132% of expected; Table
10). If, on the other hand, first female reproduction is delayed
until the second year, rmax would be 0.65 (84% of predicted;
Table 10). Procyon cancrivorus has a low Ḣb, reduced litter
size, and small body mass. Its low Ḣb may limit litter size, but
as with Bassariscus astutus, the quality of its diet (a high
percentage of small vertebrates; Table 9) and its small body
size may make it possible for females to reproduce in their first
year and thus increase the species' reproductive potential. This
reasoning would argue that Procyon cancrivorus probably
enjoys higher, rather than lower, than expected rmax.
Potos flavus.—In addition to a low Ḣb, this species possesses
other characteristics that limit its reproductive potential:
low-quality diet, delayed reproduction, and birth of a single
young each year. Because there does not appear to be any other
feature of its life history that can counteract the influence of
these factors, rmax in Potos flavus has evolved to be only 48%
of expected (0.30; Table 10). Its close relative, the olingo,
Bassaricyon gabbii, appears to share the same condition (Table
10).
Summary.—This brief survey illustrates that, with the
exception of Potos flavus, procyonids tend to have values of
rmax that are higher than those predicted for them on the basis of
mass (Table 10). Regression analysis indicates that, within the
family, body mass accounts for only a small amount (3%) of
the variation in rmax, whereas the positive slope of the
correlation between rmaxr and Hbr (R = 0.93) suggests that low
metabolism has a limiting effect on rmax (see Table 10, footnote
b). The implication here is that low Ḣb would be associated with
a lower rate of biosynthesis, a slower growth rate, and a longer
generation time. Procyonids with low Ḣb but higher than
expected rmax must possess other traits that serve to offset the
effects of low metabolism. Our survey indicates that the
following features compensate for low Ḣb and help increase
rmax: (1) a high-quality diet may make biosynthesis and growth
more efficient, thus optimizing the time element associated
[Pg 21]
with each of these processes; (2) larger litter sizes and
cooperation in care of the young may increase survivorship in
spite of a slower growth rate; and (3) an early age of first
reproduction, a long reproductive life span, and moderate-size
litters (two to four young) may in the long run add as many
individuals to the population as a shortened generation time.
Our survey also suggests that, at the other extreme, factors such
as a low-quality diet, reduced litter size, absence of cooperative
care of the young, delayed age of first reproduction, and
shortened reproductive life span all serve to decrease rmax.
Thus, it is obvious that diet, litter size, social structure,
reproductive strategy, and reproductive life span can operate
synergistically with Ḣb to magnify its influence on rmax (as with
Procyon lotor and Potos flavus), or they can function in
opposition to Ḣb to change the direction of its influence on rmax
(as with Bassariscus astutus, Procyon cancrivorus, Nasua
narica, and perhaps Nasua nasua).
Procyon lotor.—The evolution of a higher Ḣb (Tables 7, 8)
may have been the physiological cornerstone that enabled
Procyon lotor to break out of the mold being exploited by other
procyonids and to generalize its use of habitats and climates.
Once this basic physiological change was in place, selection for
appropriate alterations in thermal conductance, capacity for
evaporative cooling, diversity of diet, and energy storage would
have provided this species with the suite of adaptations needed
to extend its distribution into other habitats and climates.
Support for this concept follows from the fact that high levels
of Ḣb are associated with (1) cold-hardiness in mammals that
live in cold-temperate and arctic climates (Scholander et al.,
1950c; Irving et al., 1955; Irving, 1972:115, 116; Shield, 1972;
Vogel, 1980; Golightly and Ohmart, 1983); (2) the ability to
utilize a wide variety of food resources and to occupy a large
number of different environments and habitats (McNab,
1980a); and (3) a high intrinsic rate of natural increase (McNab,
1980a; Hennemann, 1983; Lillegraven et al., 1987; Nicoll and
Thompson, 1987; Thompson, 1987).
Other Procyonids.—Other procyonids (Potos flavus,
Procyon cancrivorus, Nasua narica, and Nasua nasua) have
lower than predicted Ḣb's (Table 7), a characteristic that is
considered to be an energy-saving adaptation for those that live
in relatively stable tropical and subtropical habitats (Müller and
Kulzer, 1977; Chevillard-Hugot et al., 1980; Müller and Rost,
1983). However, Bassariscus astutus is found in tropical,
subtropical, and temperate climates. This species is found from
tropical Mexico to temperate regions of the western United
States (Kaufmann, 1982, 1987; Nowak and Paradiso,
1983:979). In the northern part of its distribution, Bassariscus
astutus lives in habitats that are unstable (arid regions), that are
low in productivity, and that characteristically have marked
seasonal changes in temperature. Its lower than predicted Ḣb
could be an important water-conserving adaptation at times
when temperatures are high (McNab and Morrison, 1963;
McNab, 1966; MacMillen and Lee, 1970; Noll-Banholzer,
1979) and an important energy-conserving mechanism when
cold weather may limit food availability and hunting time
(Scholander et al., 1950c; Wang et al., 1973). As will be seen
later, Bassariscus astutus is unique among procyonids with
lower than predicted Ḣb's in that it also has a lower than
predicted Cmw (Table 7). This allows it to use less energy than
expected for thermoregulation at low temperatures. Another
species with a similar set of adaptations (lower than predicted
Ḣb and Cmw) is the arctic hare, Lepus arcticus (Wang et al.,
1973), which lives in one of the coldest and least-productive
regions on earth. Wang et al. (1973) suggest that this
combination of adaptations allows Lepus arcticus to better
match its energy requirements to the low productivity of its
environment. A similar relationship may hold for Bassariscus
astutus, particularly in colder arid portions of its distribution,
and may be the reason that it, but not other procyonids with low
Ḣb's, has been able to inhabit temperate climates.
Thermal conductance is a measure of the ease with which
heat is passively transferred to or from a body through its
tissues and pelt. Within Tn, a mammal is able to vary its thermal
conductance over a wide range of values by changing heat
transfer characteristics of both of these layers. Minimum
thermal conductance occurs when total heat transfer through
these layers is reduced to its lowest possible rate. This
minimum value, which is the reciprocal of maximum resistance,
occurs, theoretically, but not always practically (see
McNab, 1988b), at the animal's Tlc and is best estimated under
standard conditions in a metabolism chamber (McNab, 1980b;
Aschoff, 1981). Minimum thermal conductance scales to body
mass (McNab and Morrison, 1963; Herreid and Kessel, 1967;
McNab, 1970, 1979b; Bradley and Deavers, 1980; Aschoff,
1981). Therefore, to make comparisons between species of
various sizes, we scaled out body mass by expressing Cmw as
the ratio of measured to predicted values (Cmwr; Table 7). These
ratios were used to make comparisons of heat-transfer
characteristics between species that occupy different habitats or
climates.
In summer, Tlc's of male and female Procyon lotor (Figure
2) were very similar to those of other procyonids (22°C-26°C;
Table 7). In winter, Tlc of both sexes shifted downward to 11°C
(Figure 3). This seasonal shift in Tlc occurred as the result of a
seasonal change in minimum thermal conductance (Table 3).
For many northern mammals, a seasonal change in thermal
conductance is partly mediated via cyclic changes in the
insulative quality of their pelt (Scholander et al., 1950a; Irving
et al., 1955; Hart, 1956, 1957; Irving, 1972:165).
[Pg 22]
Procyon lotor begins to shed its heavy winter coat about the
time its young are born. Molt progresses through summer and
by late August the new coat is complete (Stuewer, 1942).
During its summer molt, Procyon lotor's Cmw increased by
about 49% over the value for female raccoons in winter (Table
3). In summer, therefore, it had the highest mass specific Cmw
of those procyonids considered (Cmwr = 1.77 and 1.79; Table 7).
An increase in thermal conductance facilitates passive heat loss
for temperate and arctic species, and this serves as an important
thermoregulatory adaptation during warm summer months
(Scholander et al., 1950c; Irving et al., 1955; Hart, 1956, 1957;
Irving, 1972:165). This adaptation is particularly important to
those temperate- and arctic-zone species (including raccoons)
whose Ḣb's do not decrease during summer (Irving et al.,
1955). From August on, the fur of Procyon lotor becomes
increasingly longer and heavier, with peak, or prime, condition
occurring in late fall and early winter (Stuewer, 1942).
Minimum conductance of our captive raccoons was lowest in
winter (Cmwr = 1.15) when their pelts were in prime condition
(Tables 3, 7). Because "primeness" of raccoon pelts varies
geographically, thicker pelts being associated with colder
climates (Goldman, 1950:21; Whitney and Underwood,
1952:24-41), the degree of seasonal change in Cmw must also
vary geographically.
The only other procyonid for which a seasonal molt has been
described is Bassariscus astutus. Molt in this species extends
from late summer to late fall (Toweill and Toweill, 1978). How
molt effects thermal conductance in Bassariscus astutus is not
known because metabolic data for this species (Table 7)
apparently were collected only when their pelts were in prime
condition (Chevalier, 1985).
Goldman (1950:20) reports that Procyon cancrivorus does
not have a seasonal molt. Like other tropical procyonids,
Procyon cancrivorus lives in an environment that has the
following characteristics: high even temperatures throughout
the year (1°C-13°C difference in monthly mean temperature),
a greater range in temperature between day and night than in
mean monthly temperature throughout the year, uniform
lengths of day and night, seasonal variation in rainfall, and
lowest temperatures during the rainy season(s) (Kendeigh,
1961:340). In such a stable environment there would be no
advantage to a sharply defined seasonal molt cycle that could
place an animal in thermoregulatory jeopardy by increasing its
thermal conductance. This would be particularly true for
animals like tropical procyonids that have lower than predicted
Ḣb's but that maintain typical eutherian body temperatures
(Table 7). Consequently, molt in all tropical procyonids may
either be prolonged or continuous. This is a feature of their
biology that needs to be examined in more detail.
Procyon lotor versus Tropical Procyonids.—Cmwr for
Procyon lotor in winter was 1.15, which is similar to the values
for Potos flavus and Procyon cancrivorus, 1.02 and 1.25,
respectively (Table 7). These two tropical species, therefore,
have Cmw's that are similar on a mass specific basis to the value
for Procyon lotor in winter. However, at their Tlc's, the thermal
gradient sustained by these tropical animals is only about 11°C,
whereas for Procyon lotor in winter it was 26.5°C. Examination
of Eq. 4 with respect to these thermal gradients suggests that
tropical procyonids achieve such low Cmw's by virtue of their
lower than predicted Ḣb's rather than by having pelts that are
exceptionally good insulators. In fact, the insulation afforded
by the pelts of these tropical procyonids is about the same as
that of the 50 g arctic lemming, Dicrostonyx groenlandicus
rubricatus, whose coat has an insulative value that is about half
that of the hare, Lepus americanus, red fox, Vulpes fulva
alascensis, and pine martin, Martes americana, animals
comparable in size to these procyonids (Scholander et al.,
1950a). Therefore, pelts of these tropical procyonids do not
have the same insulative value as the prime winter coat of
Procyon lotor.
Nasua narica and Nasua nasua have tropical and subtropical
distributions and they are the only procyonids that are diurnal
(Kaufmann, 1962:103-105, 1982, 1987). Because they are
active during the day they experience a more extreme thermal
environment (higher Ta's and solar radiation) than their
nocturnal cousins. Values of Cmwr for Nasua narica (1.45 and
1.55) and Nasua nasua (1.24 and 1.65) are higher than those
for Procyon cancrivorus or Potos flavus (Table 7). Thus, these
coatis have higher mass specific Cmw's than their nocturnal
tropical cousins. A high Cmw reduces the cost of thermoregulation
in hot environments because it increases an animal's
ability to lose excess heat passively. The higher Cmw's of these
coatis serve as an adaptation that contributes to the success of
their diurnal life style as well as their ability to expand their
habitat use to areas with less thermal stability, such as oak and
pine woodlands and deserts.
Bassariscus astutus.—This species has the lowest mass
specific Cmw of these procyonids (Cmwr = 0.85; Table 7), which
indicates that its pelt has a greater insulative value than the
coats of Potos flavus, Procyon cancrivorus, Nasua nasua, or
Nasua narica. This, coupled with a lower than predicted Ḣb,
allows Bassariscus astutus to maintain Tb with less energy
expenditure than is possible for any other procyonid of
comparable size; and this combination of adaptations provides
Bassariscus astutus with a distinct energy advantage in
environments that have low productivity (Wang et al., 1973).
The evolution of a pelt that provides better insulation must be
considered an, important contributing factor for the spread of
this species into desert regions of the western United States.
Stored Fat at Low Temperatures
Thermoregulation.—At temperatures below a mammal's
Tn, heat loss exceeds Ḣb. To maintain Tb under these
[Pg 23]
conditions, metabolic rate must be increased (Eq. 4). Procyon
lotor in summer during its annual molt (Table 5; Figure 2),
Bassariscus astutus (Chevalier, 1985), Nasua nasua (Chevillard-Hugot
et al., 1980; Mugaas et al., in prep.), Nasua narica
(Scholander et al., 1950b; Mugaas et al., in prep.), and Potos
flavus (Müller and Kulzer, 1977; Müller and Rost, 1983) all are
able to elevate their metabolic rates by 130% above basal when
they are exposed to Ta = 0°C. Procyon cancrivorus responds to
0°C with an increase in metabolic rate of 257% above basal
(Scholander et al., 1950b). All animals listed have about the
same Tlc and Tb, so the temperature differential producing this
response is about the same for each species. Metabolic ability
to defend body temperature against low ambient temperatures,
therefore, is well developed in these procyonids. Such large
increases in metabolic rate are energetically expensive, and if
these animals were routinely exposed to Ta = 0°C, it would be
difficult for them to acquire enough food each day to maintain
endothermy. Raccoons in winter pelage, however, need only
elevate their metabolic rate by 47% above basal to maintain
endothermy at Ta = 0°C (Table 5; Figure 3). Each year at the
completion of its molt, the raccoon's highly insulative pelt is
renewed. This lowers their Tlc by 9°C to 15°C below that
measured for them in summer (Figure 3) and decreases their
cost of thermoregulation at low temperatures. The increased
insulative capacity of their pelt is one of the primary
adaptations that has allowed Procyon lotor to extend its
distribution into cold climates.
Stored Fat.—Cyclic fattening is an integral and important
part of a raccoon's annual cycle (Mugaas and Seidensticker,
ms); however, it has not been reported for other procyonids.
During winter in parts of the United States and Canada,
raccoons are confined to their dens for variable periods of time
(days to months) depending on the severity of the weather
(Stuewer, 1943:223-225; Whitney and Underwood, 1952:108-116;
Sharp and Sharp, 1956; Mech et al., 1968; Schneider
et al., 1971). During this confinement, they do not hibernate but
rather enter a state of "dormancy" and become inactive. While
dormant they remain endothermic (Tb > 35°C; Thorkelson,
1972:87-90) and derive most of their energy requirement from
fat reserves accumulated during fall. The rate at which fat stores
are consumed during winter dormancy depends on the
thermoregulatory requirement imposed on them by local
weather conditions, the insulative quality of their pelt, and any
advantage they may gain by seeking shelter in a den.
Heat transfer between an animal and its environment is a
function of the interaction of its body temperature and thermal
conductance with various environmental variables (air temperature,
wind speed, vapor pressure, and thermal radiation).
When a raccoon is outside its den, its thermal conductance
(Cmw) is the only barrier to heat transfer with the external
environment. However, when it enters a tree den, a raccoon
imposes two other thermal barriers between itself and the
external environment: (1) conductance of the air space between
its fur and the den's walls (Ca) and (2) conductance of the den's
walls (Cd; Thorkelson, 1972:59-63; Thorkelson and Maxwell,
1974). Thorkelson and Maxwell (1974) modeled heat transfer
of a simulated raccoon (a water-filled aluminum cylinder
equipped with a heater and covered with a raccoon pelt) in a
closed tree den. In their system, 65% of resistance to heat flux
was attributable to the pelt, whereas the remainder (35%) was
due to Ca and Cd. Because resistance is the inverse of
conductance, and resistances for the raccoon and its den are
arranged in series, we can estimate total conductance (Ct) of
this system with Eq. 7.
| 1/Ct = 1/Cmw + 1/Ca + 1/Cd | Eq. 7 |
Minimum thermal conductance Cmw for raccoons in winter
was 0.0172 mL O2·g-1·h-1·°C-1 (Table 3). Based on Thorkelson
and Maxwell's (1974) model we let 1/Cmw = 0.65(1/Ct) = 1/0.0172 mL
O2·g-1·h-1·°C-1, and 1/Ca + 1/Cd = 0.35(1/Ct). Substituting
these values into Eq. 7 and solving for Ct yields 0.0112
mL O2·g-1·h-1·°C-1, a value that is 35% lower than that of the
animal alone. Substituting this value and the value for basal
metabolism of winter raccoons (0.47 mL O2·g-1·h-1; Table 7)
into Eq. 4 and solving for (Tb - Ta) yields a new temperature
differential of 42°C. Therefore, by using tree dens, raccoons in
north central Virginia, with Tb = 37°C (Figure 7), could
effectively reduce their Tlc from 11°C to -5°C and markedly
reduce their metabolic cost of thermoregulation.
Given prevailing winter temperatures in north central
Virginia (see "Materials and Methods"), adult raccoons in that
area should be able to sustain endothermy most of the time they
are in their dens by simply maintaining Ḣb. Depending on the
mass of their stored fat, they could remain in their dens for
several weeks without eating (Mugaas and Seidensticker, ms).
The thermal advantage of a den could be further enhanced
during colder temperatures if two or more raccoons occupied it
at the same time and huddled together, and/or if these animals
could reduce Cmw even more by lowering Tb and cooling their
extremities. Although we do not have any data to verify the
second mechanism, there are many accounts in natural history
literature that document raccoons occupying dens together
(Lotze and Anderson, 1979). This habit could be particularly
important for the young of the year and may be one reason why
they often continue to den with their mothers during winter
(Lotze and Anderson, 1979; Seidensticker et al., 1988).
Raccoons that live in colder climates, such as Minnesota,
undoubtedly obtain the same advantage from a den as Virginia
animals, but because of their greater body mass, longer fur, and
potentially lower Cmw, Tlc of a Minnesota raccoon in a den
could be even lower than what we calculated for Virginia
raccoons. Therefore, when they are in their dens, raccoons
living in very cold climates also may be able to maintain
homeothermy with a basal level of metabolism.[Pg 24]
In hot environments mammals depend on behavior to
minimize their thermal load (escape to shaded or cooler
microclimates, use posture and orientation to wind and sun,
restrict activity, become nocturnal, etc.) and on evaporative
water loss to rid themselves of excess heat. With regard to
evaporative heat loss, Calder and King (1974:326) arbitrarily
subdivided the response to various Ta's as follows: "(1) cool
temperatures at which water loss should be minimized, both to
reduce heat loss and as an adaptation to terrestriality; (2) an
intermediate temperature range wherein evaporation is gradually
increased as dry heat losses are proportionately reduced
with smaller thermal gradients; and (3) warm to hot temperatures
at which evaporation must be actively increased to
dispose of metabolic and exogenous heat loads." Some
mammals are able to thermoregulate very well at high ambient
temperatures via panting or sweating, whereas others have a
very limited capacity. Hence, there is no general approach to
calculating evaporative water loss under these conditions
(Campbell, 1977:85). However, the ratio of evaporative heat
lost to metabolic heat produced can be used to quantify a
species' capacity for evaporative cooling and to make
comparisons between species.
Potos flavus.—This species lives in Neotropical forests of
Central and South America. It is nocturnal, arboreal in habit,
and appears to be the most heat-sensitive of these procyonids.
Its Tuc is at 30°C to 33°C (Table 7; Müller and Kulzer, 1977;
Müller and Rost, 1983). It begins to pant at about 30°C, but its
efforts at evaporative cooling are very ineffective. At 33°C
Potos flavus can dissipate 33% of its metabolic heat via
evaporative water loss, but at 35°C the efficiency of this
mechanism falls to 20% (Müller and Rost, 1983). Consequently,
when exposed to Ta's above 33°C, any kind of
excitement causes its Tb to rise rapidly in an uncontrolled
manner (Müller and Kulzer, 1977; Müller and Rost, 1983).
These animals rely on their nocturnal and arboreal habits to
keep them out of situations that could lead to hyperthermia
(Müller and Kulzer, 1977; Müller and Rost, 1983).
Nasua nasua and Nasua narica.—Nasua nasua is abundant
in tropical and subtropical South America, whereas Nasua
narica occupies the same climates in North America from
southern Arizona and New Mexico south through Panama and
on into Colombia and Ecuador (Hall and Kelson, 1959:892;
Ewer, 1973:391, 392; Poglayen-Neuwall, 1975). Both coatis
are diurnal and forage primarily on the ground (Kaufmann,
1962:185-188, 1987; Poglayen-Neuwall, 1975; Nowak and
Paradiso, 1983:982), consequently they are exposed to a more
severe thermal environment while active (higher Ta's and solar
radiation) than are nocturnal procyonids. Both coatis are more
heat-tolerant than Potos flavus; their Tuc's are higher (33°C-35°C;
Table 7), they can tolerate Ta's of 35°C without raising
their Tb's (Chevillard-Hugot et al., 1980; Mugaas et al., in
prep.), and they have a greater capacity for evaporative cooling
than Potos flavus (Mugaas et al., in prep.). The greater heat
tolerance of these coatis is compatible with their diurnal habits
and widespread distribution in a variety of forest habitats in
both tropical and subtropical areas of the western hemisphere.
Bassariscus astutus.—In addition to living in Neotropical
forests of Mexico, Bassariscus astutus also flourishes in hot
arid climates, and it has extended its range much farther north
than Nasua narica (Hall and Kelson, 1959:881,892; Poglayen-Neuwall,
1975; Kaufmann, 1982). Its Tuc is higher (35.5°C;
Table 7) than that of Potos flavus, but it is comparable to those
of Nasua nasua and Nasua narica. Its capacity for evaporative
cooling is well developed; at 40°C Bassariscus astutus is able
to dissipate 100% of its resting metabolic heat via evaporative
water loss, and at 45°C it is able to dissipate 172% (Chevalier,
1985). In spite of its great capacity for evaporative cooling, this
species is nocturnal, a habit that, along with its low Ḣb, should
allow it to keep thermoregulatory water requirements to a minimum.
Procyon lotor.—Our data suggested that Tuc for Procyon
lotor in winter was comparable to that for Bassariscus astutus
(35°C), and that in summer it was even higher. When exposed
to temperatures near the upper end of its Tn, Procyon lotor
increased the gradient for passive heat loss with a controlled
rise in Tb (Figure 6). In summer its capacity for passive heat
loss was enhanced by the molt of its heavy winter fur. Procyon
lotor's capacity for evaporative cooling also appeared to be
well developed, although our animals were not heated to the
point that evaporative cooling was fully expressed (Figures 4,
5). However, Procyon lotor is nocturnal, and this may allow it
to eliminate, or at least reduce, the need for evaporative
cooling, even in hot climates. Thus, Procyon lotor appears to be
well equipped physiologically and behaviorally to cope with
thermal demands of hot environments in its distribution.
Procyon cancrivorus.—Unfortunately, data for the crab-eating
raccoon are not complete enough at high temperatures to
include it in this survey.
Summary.—This comparison demonstrates that capacity
for evaporative cooling, tolerance of an elevated Tb to enhance
passive heat loss, and behavioral avoidance of thermal stress
are the primary methods used by procyonids to thermoregulate
at high temperatures. Procyon lotor and Bassariscus astutus,
whose distributions extend into temperate regions, have
developed these abilities to a greater extent than other
procyonids. Potos flavus, whose distribution is confined to
lowland tropical forests, has the least ability in this regard.
Nasua nasua and Nasua narica appear to have thermoregulatory
abilities that are intermediate to those of Bassariscus
astutus and Potos flavus. This suggests that ancestral procyonids
[Pg 25]
may have had poor to modest ability to thermoregulate
at high temperatures, a condition that would have limited their
ability to leave the thermal stability afforded by tropical forests.
Dispersal into temperate climates, therefore, required not only
increased cold tolerance but also selective enhancement of
those mechanisms used in thermoregulation at high temperatures.
| Species | Tropics | Subtropics | Mild[a] temperate | Cold[b] temperate | ||
|---|---|---|---|---|---|---|
| Procyon lotor | + | + | + | + | ||
| Bassariscus astutus | + | + | + | |||
| Nasua nasua | + | + | ||||
| Nasua narica | + | + | ||||
| Procyon cancrivorus | + | + | ||||
| Potos flavus | + | |||||
[a] Extends from the subtropics north to the northern limit of Bassariscus astutus' distribution (Hall and Kelson, 1959:881), which approximates the 10°C isotherm for average annual temperature in the United States (Kincer, 1941).
[b] Extends northward from the 10°C isotherm for average annual temperature in the United States.
In Table 11, procyonid species are arranged in descending
order with respect to the number of major climates that are
included in their geographic distributions (Hall and Kelson,
1959:878-897; Poglayen-Neuwall, 1975; Kortlucke and Ramirez-Pulido,
1982; Nowak and Paradiso, 1983:977-985).
Composite scores ranged from a high of 1.47 for Procyon lotor
to a low of 0.39 for Potos flavus, whereas Nasua nasua, Nasua
narica, Procyon cancrivorus, and Bassariscus astutus had
intermediate values ranging from 0.64 to 0.79 (Table 12).
Figure 8 demonstrates that there is a direct relationship between
the number of climates these species occupy and their
composite scores. Regression analysis (Y = 2.68·X + 0.24;
where Y is number of climates, and X is composite score)
demonstrates a high degree of correlation between these
variables (R = 0.94) and indicates that 89% of the variance in
distribution can be explained by composite scores. The various
combinations of adaptations expressed by these species do,
therefore, play a role in delimiting their climatic (latitudinal)
distributions.
Procyon lotor's normalized scores were higher in all
categories than those of other procyonids. Procyon lotor,
therefore, possesses those traits that have allowed it to become
the premier climate generalist of the procyonid family. As an
adaptive unit, these traits provide Procyon lotor with the
physiological and behavioral flexibility required to take full
advantage of a wide range of climates and habitats, and its
distribution verifies that it has done so. Even so, it is probably
not fair to assume that this species represents a perfect
physiological match with climate over its entire distribution.
Procyon lotor is, in many respects, still a forest-dwelling
species, and its ability to expand its distribution into other
habitats such as prairie and desert may well be due, in part, to
its use of behavior to take advantage of favorable microclimates
in otherwise hostile environments (Bartholomew, 1958,
1987). This feature of Procyon lotor's biology needs to be
further examined.
ratio of measured to predicted basal metabolism (Table 7), Cmwr = ratio of
measured to predicted minimum thermal conductance (Table 7), Ddr = ratio of
food categories actually utilized by each species to total food categories eaten
by all six species (calculated from Table 9), rmaxr = ratio of calculated to
expected rmax (Table 10).)
| Species | Normalized scores | Composite[a] score | |||
|---|---|---|---|---|---|
| Hbr/Cmwr | Ddr | rmaxr | |||
| Procyon lotor | 0.95 | 0.95 | 2.52 | 1.47 | |
| Bassariscus astutus | 0.80 | 0.33 | 1.24 | 0.79 | |
| Nasua nasua | 0.48 | 0.33 | 1.11[b] | 0.64 | |
| Nasua nasua | 0.48 | 0.33 | 1.11[b] | 0.64 | |
| Nasua narica | 0.40 | 0.53 | 1.11 | 0.68 | |
| Procyon cancrivorus | 0.55 | 0.33 | 1.32 | 0.73 | |
| Potos flavus | 0.60 | 0.11 | 0.48 | 0.39 | |
[a] Composite score = [(Hbr/Cmwr) + Ddr + rmaxr]/3.
[b] Value calculated for Nasua narica (Table 10) and used with the assumption that it must be similar to the value for Nasua nasua.
All five species with low Ḣb's have composite scores less
than 1.0 (Table 12; Figure 8). Four of these five, Nasua nasua,
Nasua narica, Procyon cancrivorus, and Potos flavus, have
Hbr/Cmwr ratios that are 0.6 or less, which indicates
they are the least cold-tolerant procyonids (McNab, 1966).
These four species also are confined to either tropic, or tropic
and subtropic climates (Table 11). This suggests that these
species share a common thermoregulatory adaptation that
represents a specialization to these climates. Attendant with
this adaptation, however, is a high cost of thermoregulation at
[Pg 26]
temperatures below their Tlc, and this must be an important
factor in limiting their distributions to tropic and subtropic
climates. Differences in their distributions within these
climates, therefore, must hinge more on differences in their Ddr
and rmaxr values than on differences in their Hbr/Cmwr ratios.
This is supported by the fact that Potos flavus, which has the
lowest Ddr and rmaxr values, is confined to a single climate,
whereas Nasua nasua, Nasua narica, and Procyon cancrivorus
each possess larger Ddr and rmaxr values and are found
in two climates. Thus, Potos flavus, with its highly specialized
diet and low reproductive potential, is the most ecologically
specialized of these procyonids, and its distribution is limited to
the single climate that can provide its requirements. Nasua
nasua, Nasua narica, and Procyon cancrivorus are less
specialized and thus show more ecological flexibility in their
distributions.
Bassariscus astutus, the other species with low Ḣb, is found
in three climates, which indicates that it has greater ecological
flexibility than Nasua nasua, Nasua narica, or Procyon
cancrivorus. Ddr and rmaxr are comparable for these four species
(Table 12). This suggests that the greater ecological flexibility
of Bassariscus astutus is derived largely from its greater cold
tolerance. Bassariscus astutus has a more insulative pelt than
these other procyonids (Cmwr = 0.85; Table 7), so its Hbr/Cmwr
ratio is higher (0.80; Table 12). This, and its greater capacity for
evaporative cooling (Chevalier, 1985), allows Bassariscus
astutus to take advantage of a wider range of thermal
environments than these other species. However, even with its
higher Hbr/Cmwr ratio, the composite score for Bassariscus
astutus is not much different than those for Nasua nasua,
Nasua narica, and Procyon cancrivorus (Table 12). Consequently,
Bassariscus astutus is found in more climates than
would be predicted for it on the basis of its composite score
(Figure 8). This suggests that either the Hbr/Cmwr ratio carries
greater weight in determining distribution than is reflected in
this analysis, or as has been described for some other species
(Bartholomew, 1958, 1987), Bassariscus astutus may extend
its distribution farther than expected via use of its behavior. In
either case, for procyonids with low Ḣb, Bassariscus astutus
represents the pinnacle of adaptation for climate generalization.
A radiation of frugivorous and omnivorous Procyoninae
(Table 1) occurred in the middle and late Miocene of North
America. It included origins of such terrestrial genera as
Cyonasua, Nasua, and Procyon (Webb, 1985b). The earliest
procyonid genus to find its way to South America was
Cyonasua, an omnivorous carnivore that presumably split,
along with its sister genus Arctonasua, from a common North
American ancestor (Baskin, 1982; Webb, 1985b). Cyonasua,
about the size of present-day raccoons, was adapted to a wide
range of habitats and was probably comparable to modern
raccoons with respect to the breadth of its feeding habits
(Webb, 1985b; Marshall, 1988). Because North American
Arctonasua was about the same size as Cyonasua (Webb,
1985b) and shared a number of characters with it (Baskin,
1982), we speculate that it also may have had similar habits and
occupied similar climates and habitats. Bassariscus, another
member of Procyoninae, had an even earlier origin in tropical
North America (Webb, 1985b). The origin of the small arboreal
forms Potos and Bassaricyon (subfamily Potosinae) is obscure
but is thought to have occurred in the rainforests of Central
America (Webb, 1985b). What were the metabolic capabilities
of these early procyonids? We do not know, but for several
million years, from middle to late Miocene, procyonids lived in
tropical and subtropical forests of Central and North America
(Webb, 1985b; Marshall, 1988). Then, in the Pleistocene,
several modern forms crossed the Panamanian land bridge into
similar habitats and climates in South America; but none of
them appear to have spread far enough northward to have
crossed the Bering land bridge.
Several million years exposure to a tropical environment,
with its continuous high temperatures and modest range of
thermal extremes, would have favored selection of metabolic
and thermoregulatory traits that would minimize energy
requirements: a lower than predicted basal metabolic rate, a
prolonged or continuous molt resulting in very little annual
change in minimum thermal conductance, and a modest
capacity for evaporative cooling. In addition, we would expect
selection to have favored a diverse diet, good reproductive
[Pg 27]
potential, and behavioral flexibility to utilize a variety of
habitats within these climates. Our analysis has shown that
such characteristics are the norm for extant members of this
family living in tropical and subtropical climates, and we
speculate that these traits also were common to early
procyonids and served to restrict them to these climates. Our
speculation is supported by the fact that their known fossil
history from the Miocene is confined to geographic areas that
had tropical and subtropical climates.
Later on, during Pleistocene glaciations, tropical and
subtropical forests shrank, savannas expanded, and temperate
climate was pushed toward equatorial regions. The opposite
occurred during interglacial periods (Raven and Axelrod, 1975;
Webb, 1977, 1978; Marshall, 1988). Consequently, mid-latitudes
experienced alternating periods of temperate and
tropical, or at least subtropical, climate change. Selection of
characteristics that would have adapted a species with low Ḣb
to temperate as well as tropic or subtropic climates could have
occurred in mid-latitudes at the temperate edge of these tropical
advances and retreats. Our analysis indicates that, for this
purpose, selection would have favored lower than predicted
thermal conductance, seasonal molt, increased capacity for
evaporative cooling, increased tolerance of elevated Tb,
increased flexibility of thermoregulatory behavior, food habits
that provided for year-round access to a high-quality diet in all
three climates, and a higher than predicted rmax.
Bassariscus astutus is the only species with low Ḣb that has
all these characteristics, and it is the only one of them that has
added temperate climate to its distribution (Table 11). This
suggests that Bassariscus astutus is a species that evolved away
from the norm for procyonids with low Ḣb, toward characteristics
that allowed it to become more of a climate generalist.
Potos flavus, with its dietary specialization, low tolerance to
high temperatures, and arboreal mode of existence, has become
a highly specialized species totally dependent on tropical
forests for its survival. As such, it also represents a species that
has evolved away from the procyonid norm and portrays the
extreme in climate specialization. Olingos, Bassaricyon gabbii
(Table 1), may be similar to Potos flavus in this respect (see
also Table 10). This suggests that of the extant procyonids,
Nasua nasua, Nasua narica, and Procyon cancrivorus have
retained metabolic and behavioral characteristics that are
closest to those of their Miocene ancestors.
Between the time that Cyonasua appeared and the Panamanian
land bridge was established in the upper Pliocene (4 to 5
million years ago), northern climates continued their gradual
cooling. This, along with ongoing elevation of the continents
and continuous modification of their mountain ranges, served
to shrink the tropical forest and create pockets of climatic
instability within it and on its edges (Darlington, 1963:578-596;
Marshall, 1988). In areas of instability, selection would
have favored traits that provided for a broader range of thermal
tolerance: higher Ḣb, improved insulative quality of pelt, a
more sharply defined molt cycle, improved capacity for
evaporative cooling, greater Dd, and higher rmax. Consequently,
by the upper Pliocene, two metabolically distinct groups of
procyonids could have been established: those species with low
Ḣb living in climatically stable forests and those with higher Ḣb
living in unstable tropical, subtropical, and perhaps temperate
climates.
Procyon lotor is the only extant procyonid with high Ḣb.
Procyon cancrivorus is its congeneric counterpart in Central
and South America (Table 1), and the two species are sympatric
in Panama and Costa Rica. However, in terms of its
metabolism, thermal conductance, molt, diversity of diet, rmax,
and climatic distribution, Procyon cancrivorus shares more in
common with other procyonids than it does with Procyon lotor
(Tables 7, 11, 12; Figure 8). This suggests that metabolically
Procyon lotor portrays a divergent line of this genus that arose
as the result of a series of mutations that gave rise to different
metabolic characteristics. This view is in keeping with a recent
phylogenetic analysis of this family that shows the genus
Procyon to be highly derived (Decker and Wozencraft, 1991).
Consequently, it would be instructive and would add to our
knowledge of the evolution of climatic adaptation to know
more about the genetic relatedness of these two species as well
as their historical relationship.
Genus Procyon appears in the fossil record (Hemphillian and
Blancan ages; Baskin, 1982) prior to Pleistocene glaciations.
During the Pleistocene, there were four different glacial
advances and retreats in a relatively short time period (the first
appearing little more than a million years ago; Darlington,
1963:578-596; Webb, 1985a; Marshall, 1988). Glacial retreats
created pulses of time during which subtropic and temperate
climates advanced toward the poles into areas with large
seasonal differences in light/dark cycles, whereas glacial
advances pushed these climates southward into areas having
smaller seasonal differences in light/dark cycles (Raven and
Axelrod, 1975; Webb, 1977, 1978; Marshall, 1988). Those
members of the genus Procyon caught in these wide latitudinal
fluctuations would have experienced conditions favorable to
continued selection for characteristics conducive to physiologic
adaptation to a wide range of climatic conditions. Procyon
lotor is the only member of its genus to have survived this
selective process, and as we have seen, it does possess traits
that adapt it to a wide range of climatic conditions. Primary
among these is its higher Ḣb, which provides it with advantages
not shared with other procyonids (see earlier discussion). Three
other adaptations also have had a profound influence on
Procyon lotor's ability to generalize its use of climate: (1) the
increased insulative quality of its pelt coupled with its sharply
defined molt cycle, which allows for a large annual change in
thermal conductance; (2) its annual cycle of fat storage; and (3)
a diverse high-quality diet. The first two of these adaptations
[Pg 28]
required evolution of neuroendocrine pathways capable of
responding to time-dependent environmental cues such as
changing day length, changing temperature, etc. Such conditions
would have been available as selective stimuli in
high-latitude forests and savannas of interglacial periods.
Procyon lotor's elevated basal metabolic rate would have
increased its overall energy requirement, and it makes good
intuitive sense, therefore, that evolution during the Pleistocene
also would have favored selection of a diverse diet containing
many items of high nutritive value.
Our analysis has illustrated that within Procyonidae there are
two distinct modes of metabolic adaptation to climate. One is
typified by those species with low Ḣb's (Bassariscus astutus,
Nasua nasua, Nasua narica, Procyon cancrivorus, and Potos
flavus), and the other by Procyon lotor with its higher Ḣb.
Those with low Ḣb's have more restricted geographic distributions,
and, with the exception of Bassariscus astutus, they are
all confined to tropical and subtropical areas. The fossil history
of this family indicates that it had its origins in tropical forests
of North and Central America. This indicates that those
procyonids whose distributions are still primarily restricted to
tropical forests share many of the metabolic adaptations
characteristic of their ancestors. We speculate, therefore, that
ancestral procyonids had a lower than predicted Ḣb, a pelt with
modest to poor insulative quality, good thermogenic ability but
poor heat tolerance, modest to poor capacity for evaporative
cooling, no well-defined molt cycle, no cyclic period of
fattening, nocturnal habits, and a modestly diverse diet of
high-enough quality to provide for an average reproductive
potential. Although this pedigree contributed to the success of
this family in tropical and subtropical forests, it limited the
ability of its members to expand their distributions into cooler,
less stable climates. Viewed in this perspective, Procyon
lotor's high basal metabolic rate, extraordinarily diverse diet,
well-defined cyclic changes in fat content and thermal
conductance, high level of heat tolerance, high capacity for
evaporative cooling, and high reproductive potential all stand
out in sharp contrast to the condition described for other
procyonids. This suggests that the North American raccoon
represents culmination of a divergent evolutionary event that
has given this species the ability to break out of the old
procyonid mold and carry the family into new habitats and
climates.
[↑ TOC]
[Pg 29]
| a | potential age of females first producing young |
| b | potential annual birth rate of female young |
| Ca | conductance of air |
| Cd | conductance of den walls |
| Cm | minimum thermal conductance |
| Cmd | minimum dry thermal conductance |
| Cmw | minimum wet thermal conductance |
| Cmwr | ratio of measured to predicted minimum wet thermal conductance |
| Ct | total conductance |
| Dd | diversity of diet |
| Ddr | ratio of food categories actually used by a species to the total number of food categories taken by all species tested |
| Ė | evaporative water loss |
| Ec | ratio of evaporative heat lost to metabolic heat produced |
| Ėeq | oxygen equivalent for heat lost by evaporation |
| Ḣb | basal metabolic rate |
| Ḣr | lowest resting metabolic rate at each temperature |
| Hbr | ratio of measured to predicted basal metabolic rate |
| m | mass of animal |
| mw | mass of water |
| n | potential age of females producing their final young |
| rmax | intrinsic rate of natural increase |
| rmaxe | expected intrinsic rate of natural increase |
| rmaxr | ratio of calculated to expected intrinsic rate of natural increase |
| RQ | respiratory quotient |
| Ta | chamber air temperature |
| Tb | body temperature |
| Tlc | lower critical temperature |
| Tn | thermoneutral zone |
| Tuc | upper critical temperature |
| t | time |
| .Va | rate of air flow through |
| .Ve | rate of air flow into metabolism chamber |
| α | active phase of the daily cycle |
| γ | heat equivalent of oxygen |
| λ | heat of vaporization of water |
| ρ | rest phase of the daily cycle |
[Pg 30]
[↑ TOC]
Aschoff, Jürgen
Aschoff, J., and H. Pohl
Barghoorn, Elso S.
Bartholomew, George A.
Baskin, Jon Alan
Benedict, Francis G.
Berggren, William A.
Bisbal, Francisco J.
Bradley, S. Robert, and Daniel R. Deavers
Brody, Samuel
Calder, William A., III
Calder, William A., and James R. King
Campbell, Gaylon S.
Chevalier, C. D.
Zoologist, 25:58A.
Chevillard-Hugot, Marie-Christine, E. F. Müller, and E. Kulzer
Colbert, Edwin H.
Cole, Lamont C.
Crandall, Lee S.
Crockett, Curtis W.
Darlington, Philip J., Jr.
Davis, D. Dwight
Decker, Denise M.
Decker, Denise M., and W. Chris Wozencraft
Depocas, Florent, and J. Sanford Hart
Dunn, J. P., and J. A. Chapman
Eisenberg, John F.
Ewer, R. F.
Fenchel, Tom
Ford, Linda S., and Robert S. Hoffmann
Ginsburg, Léonard
[Pg 31]
6:247-258, 12 figures.
Glazier, Douglas S.
Goldman, Edward A.
Golightly, Richard T., Jr., and Robert D. Ohmart
Greenwood, Raymond J.
Hall, E. Raymond, and Keith R. Kelson
Hallett, James G., Margaret A. O'Connell, Gregory D. Sanders, and John Seidensticker
Hamilton, W. J., Jr.
Hart, J. S.
Hart, J. S., and O. Heroux
Hayssen, V.
Hemmingsen, Axel M.
Hennemann, Willard W., III
Herreid, Clyde F., II, and Brina Kessel
Hinds, David S.
Hulbert, A. J., and T. J. Dawson
Hunt, Robert M., Jr.
Irving, Laurence
Irving, Laurence, Hildur Krog, and Mildred Monson
Kaufmann, John H.
Kendeigh, S. Charles
Kincer, J. B.
King, James R.
Kleiber, Max
Kortlucke, S., and J. Ramirez-Pulido
Lasiewski, Robert C, and Roger S. Seymour
Leone, Charles A., and Alvin L. Wiens
List, Robert J.
Smithsonian Miscellaneous Collections, 114: xi + 527 pages, 174 tables.
Lotze, Joerg-Henner, and Sydney Anderson
Lusk, Graham
MacMillen, Richard E., and Anthony K. Lee
Marshall, Larry G.
Martin, Alexander C, Herbert S. Zim, and Arnold L. Nelson
Martin, Larry D.
McNab, Brian K.
McNab, Brian K., and Peter Morrison
Mech, L. David, Donald M. Barnes, and John R. Tester
Mellen, William J.
Agricultural Science Review, Fall:20-26, and 49, 1 figure.
Mugaas, John N., and James R. King
Mugaas, John N., and John Seidensticker
Mugaas, John N., John Seidensticker, and Paul Cook
Müller, E., and E. Kulzer
Müller, E. F., and H. Rost
Nicoll, M. E., and Steven D. Thompson
Noll-Banholzer, Ursel
Nowak, Ronald M., and John L. Paradiso
Ott, Lyman
Poglayen-Neuwall, I.
Poglayen-Neuwall, I., and Ingeborg Poglayen-Neuwall
(Liechtenstein, 1830). Zeitschrift für Saügetierkunde, 45:73-81, 1 figure.
Poglayen-Neuwall, Ivo, and Dale E. Toweill
Prosser, C. Ladd
Prothero, John
Raven, Peter H., and Daniel I. Axelrod
Robbins, Charles T.
Russell, James K.
Sanderson, G. C.
Sarich, V. M.
Schmitz, O. J., and D. M. Lavigne
Schneider, Dean G., L. David Mech, and John R. Tester
Scholander, P. F., Vladimir Walters, Raymond Hock, and Laurence Irving
Scholander, P. F., Raymond Hock, Vladimir Walters, Fred Johnson, and Laurence Irving
Scholander, P. F., Raymond Hock, Vladimir Walters, and Laurence Irving
Segall, Walter
Seidensticker, John, A. J. T. Johnsingh, Rebecca Ross, Greg Sanders, and Maryla B. Webb
Sharp, Ward M., and Louise H. Sharp
Shield, John
Shkolnik, Amiram, and Knut Schmidt-Nielsen
Stains, Howard J.
Statistical Analysis System (SAS)
Stuewer, Frederick W.
Tagle, D. A., M. M. Miyamoto, M. Goodman, O. Hofmann, G. Braunitzer, R. Göltenboth, and H. Jalanka
Taylor, Walter P.
Thompson, S. D.
Thorkelson, Jeffrey
Thorkelson, Jeffrey, and Robert K. Maxwell
Todd, Neil B., and Suzanne R. Pressman
2 figures.
Toweill, Dale E., and James G. Teer
Toweill, Dale E., and Deyanne B. Toweill
Trapp, Gene R.
United States Department of the Interior Geological Survey
Vogel, Peter
Wang, Lawrence C. H., Douglas L. Jones, Robert A. MacArthur, and William A. Fuller
[Pg 34]
the Arctic Hare (Lepus arcticus). Canadian Journal of Zoology, 51:841-846, 1 figure, 2 tables.
Wayne, Robert K., Raoul E. Benveniste, Dianne N. Janczewski, and Stephen J. O'Brien
Webb, S. David
Whitney, Leon F., and Acil B. Underwood
Wood, John E.
Wozencraft, W. Chris
Wurster, D. H., and K. Benirschke
Zervanos, Stam M.
[Pg 0]
Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first
Secretary of the Smithsonian. In his formal plan for the institution, Joseph Henry outlined a
program that included the following statement: "It is proposed to publish a series of reports,
giving an account of the new discoveries in science, and of the changes made from year to year
in all branches of knowledge." This theme of basic research has been adhered to through the
years by thousands of titles issued in series publications under the Smithsonian imprint,
commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the
following active series:
Smithsonian Contributions to Anthropology
Smithsonian Contributions to Botany
Smithsonian Contributions to the Earth Sciences
Smithsonian Contributions to the Marine Sciences
Smithsonian Contributions to Paleobiology
Smithsonian Contributions to Zoology
Smithsonian Folklife Studies
Smithsonian Studies in Air and Space
Smithsonian Studies in History and Technology
In these series, the Institution publishes small papers and full-scale monographs that report
the research and collections of its various museums and bureaux or of professional colleagues
in the world of science and scholarship. The publications are distributed by mailing lists to
libraries, universities, and similar institutions throughout the world.
Papers or monographs submitted for series publication are received by the Smithsonian
Institution Press, subject to its own review for format and style, only through departments of the
various Smithsonian museums or bureaux, where the manuscripts are given substantive review.
Press requirements for manuscript and art preparation are outlined on the inside back cover.
Robert McC. Adams
Secretary
Smithsonian Institution
With the exception of the typographical corrections listed below and some minor
changes that may have been made in moving tables or illustrations so that they
are rejoined, the text presented is that published in the original printed media.
| Page ii, LOC Data | : | Instituion's | => | Institution's |
| Page 1, Introduction | : | linages | => | lineages |
| Page 4, The Atypical Procyonid | : | consumate | => | consummate |
| Page 21,Summary | : | Table 10, footnote f | => | Table 10, footnote b |
| Page 26, first paragraph | : | Nassua | => | Nasua |
| Page 31, Literature Cited | : | Incoporated | => | Incorporated |
| Page 34, Literature Cited | : | Gettleman | => | Gittleman |
Featured Books

The Strange Case of Dr. Jekyll and Mr. Hyde
Robert Louis Stevenson
not crossed the doors of one for twentyyears. But he had an approved tolerance for others; sometimes...

Wild Birds in City Parks
Herbert Eugene Walter and Alice Hall Walter
ncoln Park.We wish to thank our friends for their kind support infurthering our efforts to enlarge t...

A New Pocket Mouse (Genus Perognathus) from Kansas
E. Lendell Cockrum
i, new subspeciesType.—Female, adult, skin and skull; No. 11716, Univ. Kansas Mus. Nat.Hist.; Cona...

She
H. Rider Haggard
istory, and then go on to tell how it found itsway into my hands.Some years ago I, the editor, was s...

The Young Acrobat of the Great North American Circus
Jr. Horatio Alger
young people had been looking forward to the advent ofthis marvelous aggregation of curiosities, an...

Cast Upon the Breakers
Jr. Horatio Alger
A MINISTER’S GOOD FORTUNE. CHAPTER XXIX. -- A MINING TOWN IN MONTANA. CHAPTER XXX. -- THE MYSTER...

The Wit and Humor of America, Volume III. (of X.)
kland W. Gillilan542MaxiomsCarolyn Wells424Morris and the Honorable TimMyra Kelly488Mr. Stiver's Hor...

All Around the Moon
Jules Verne
TRATIONS.1. HIS FIRST CARE WAS TO TURN ON THE GAS2. DIANA AND SATELLITE3. HE HELPED ARDAN TO LIFT BA...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Metabolic Adaptation to Climate and Distribution of the Raccoon Procyon Lotor and Other Procyonidae" :