University of Kansas Publications
Museum of Natural History
Volume 14, No. 6, pp. 99-110, 1 fig.
December 29, 1961
Natural History of the Brush Mouse
(Peromyscus boylii) in Kansas
With Description of a New Subspecies
BY
CHARLES A. LONG
University of Kansas
Lawrence
1961
University of Kansas Publications,
Museum of Natural History
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Theodore H. Eaton, Jr.
Volume 14, No. 6, pp. 99-110, 1 fig.
Published December 29, 1961
University of Kansas
Lawrence, Kansas
PRINTED BY
JEAN M. NEIBARGER, STATE PRINTER
TOPEKA, KANSAS
1961
28-8518
[Pg 101]
Natural History of the Brush Mouse
(Peromyscus boylii) in Kansas
With Description of a New Subspecies
BY
CHARLES A. LONG
In order to determine the geographic distribution of the brush
mouse in the state, 15 localities, chosen on the basis of suitable
habitat, were investigated by means of snap-trapping in the winter
and spring of 1959, spring of 1960, and winter and spring of 1961.
Variation in specimens obtained by me and in other specimens in
the Museum of Natural History, The University of Kansas, was
analyzed. Captive mice from Cherokee County, Kansas, were
observed almost daily from March 27, 1960, to June 1, 1961. Captive
mice from Chautauqua and Cowley counties were studied
briefly. Contents of 38 stomachs of brush mice were analyzed, and
diet-preferences of the captive mice were studied. Data from
live-trapping and from snap-trapping are combined and provide
some knowledge of size and fluctuation of populations in the species.
Examination of the accumulated specimens and the captive mice
reveals the occurrence in southern Kansas of an unnamed subspecies,
which may be named and described as follows:
Peromyscus boylii cansensis new subspecies
Type.—Male, adult, skin and skull; No. 81830, K. U.; from 4 mi. E Sedan,
Chautauqua County, Kansas; obtained on December 30, 1959, by C. A. Long,
original No. 456.
Range.—Known from 3 mi. W Cedar Vale, in Cowley County, Kansas, and
from the type locality.
Diagnosis.—Size medium (see Table 1 beyond); underparts white; upper
parts Ochraceous-Tawny laterally, becoming intermixed with black and approaching
Mummy Brown dorsally (capitalized color terms after Ridgway,
1912); eye nonprotuberant; tail short but well-haired distally and usually less
than half total length; nasals long; cranium large.
Comparisons.—From P. b. attwateri, the subspecies geographically
nearest cansensis, the latter can be easily distinguished by
the less protuberant eyes and relatively shorter tail (91 per cent
of length of head and body; in topotypes of P. b. attwateri from
Kerr County, Texas, 104 per cent; in specimens of P. b. attwateri
from Cherokee County, Kansas, 103 per cent). P. b. cansensis is
darker than P. b. attwateri and darker than P. b. rowleyi, the palest[Pg 102]
subspecies of brush mouse, which occurs to the westward. The
skull and nasals (see Table 1) in adults of P. b. attwateri from
Cherokee County average shorter than in cansensis.
Specimens examined.—Total, 26. Cowley Co.: 3 mi. W Cedar Vale, 16.
Chautauqua Co.: type locality, 10.
Table 1. Average and Extreme Measurements of Specimens of P. b.
cansensis, of P. b. Attwateri From Cherokee County, Kansas, and of
Topotypes of P. b. attwateri Listed by Osgood, 1909.
|
P. b. cansensis |
P. b. attwateri |
Three miles west
of Cedar Vale |
Type locality |
Both localities |
Two miles south
of Galena |
Type locality[A] |
No. specimens |
11 |
7 |
18 |
20 |
10 |
Total length |
180.5
170-199 |
176.7
166-188 |
179.1
..... |
186.2
170-210 |
196.0
..... |
Tail-vertebrae |
85.5
72-101 |
85.0
75-93 |
85.3
..... |
94.5
83-104 |
100.0
..... |
Hind foot |
23.1
22-24 |
23.6
22-25 |
23.3
..... |
23.8
22-25 |
21.0
..... |
Ear from notch |
18.2
17-19 |
19.1
18-21 |
18.5
..... |
18.4
14-21 |
.....
..... |
Greatest length of skull |
27.9
26.8-29.0 |
28.3
27.9-28.9 |
28.1
..... |
27.8
26.6-29.1 |
.....
..... |
Length of nasals |
10.4
9.9-10.8 |
10.2
9.5-10.7 |
10.3
..... |
9.9
9.1-10.4 |
.....
..... |
Zygomatic breadth |
14.3
13.9-15.0 |
13.5
13.0-13.9 |
13.9
..... |
13.8
13.3-14.4 |
.....
..... |
Distribution of Peromyscus boylii in Kansas
The subspecies Peromyscus boylii attwateri is known in the state
only from Cherokee County, the southeasternmost county in the
state. Probably the only locality where the brush mouse occurs in
that county is on the systems of cliffs along Shoal Creek, southward
from Galena, to the eastward of Baxter Springs. This is the extent
of the known range, and in my opinion the probable range, of P. b.
attwateri in the state (see Fig. 1). Cockrum (1952:fig. 49) by
mistake mapped the species from west of Baxter Springs in Cherokee
County.
[Pg 103]
Osgood (1909:149) recorded the subspecies P. b. attwateri from
Cedar Vale, Chautauqua County, Kansas, but the specimen from
there must now be assigned to cansensis on geographic grounds.
Probably the specimen was not obtained from Cedar Vale itself for
the habitat is not suitable there. Numerous specimens are known
from 3 mi. W Cedar Vale, in Cowley County, Kansas, all of which
are assigned to cansensis. Osgood's recorded locality is situated
between this locality and the type locality of cansensis, which is
4 mi. E Sedan, Chautauqua County, Kansas. The distribution of
cansensis also is shown in Fig. 1.
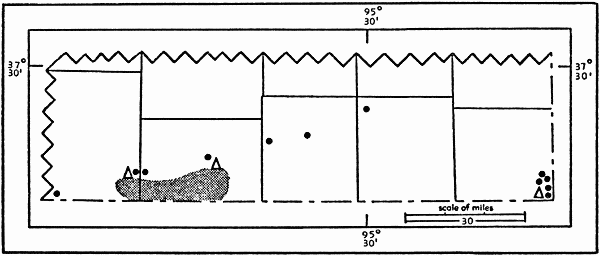
Fig. 1. Distribution of the brush mouse in Kansas. The southernmost
row of counties includes from left to right Cowley, Chautauqua, Montgomery,
Labette, and Cherokee. Black dots represent trapping localities
from which brush mice were not obtained. Triangles represent localities
from which brush mice were obtained. The stippled area contains suitable
habitat for the brush mouse, but was not investigated. The
easternmost triangle represents a place 2 mi. S Galena, Cherokee Co.,
Kansas, from which P. b. attwateri is known. The westernmost triangle
represents a place 3 mi. W Cedar Vale, in Cowley Co., Kansas, from
which P. b. cansensis is known. The triangle of intermediate position
represents the type locality of P. b. cansensis, a place 4 mi. E Sedan,
Chautauqua Co., Kansas. Many of the trapping localities have been
investigated more than once.
The probable geographic range of P. boylii is based on trapping
data (see Fig. 1). The brush mouse is confined to systems of
wooded cliffs in Kansas. The two subspecies seem to be separated
by more than 80 miles of grasslands. Blair (1959) has postulated
that in the northeastern part of its range P. b. attwateri is represented
by disjunct, relict populations formed by diminishing montane
or cool, moist environmental conditions. He has implied that
the critical climatic change occurred during post-Wisconsin times,
and that the isolation of these populations occurred so recently that
no morphological differentiation has resulted in them. Inasmuch
as the species is widely distributed in México, the southwestern[Pg 104]
United States, and in California, and has been recorded from the
Pleistocene of California (Hay, 1927:323), it is reasonable to suppose
that the species immigrated into Kansas from the southwest
and that the immigration was in a generally northward or eastward
direction. If long tail and large eyes are specializations for a
scansorial mode of life (discussed below), then P. b. cansensis must
be considered more primitive than P. b. attwateri for the eyes are
less protuberant and the tail is shorter in P. b. cansensis than in the
latter. I suggest that P. b. cansensis occurred in what is now known
as Kansas before P. b. attwateri entered this area by way of the
Ozark Mountains. The occurrence of a mouse of "the truei or
boylei group" (Hibbard, 1955:213) in southwestern Kansas in the
Jinglebob interglacial fauna of the Pleistocene adds little to support
the thesis outlined above, but is not inconsistent with the thesis.
Incidentally, the geographic distribution of P. boylii may differ
somewhat from that shown by Blair (1959:fig. 5); whereas he has
mapped the distribution of P. boylii to show disjunctivity in P. b.
attwateri and homogeneity in the distribution of other subspecies
of the brush mouse to the westward and southward, disjunctivity
actually occurs frequently also in the western and southern subspecies.
Ecology
In Kansas the brush mouse is confined to systems of cliffs, the
faces of which range in height to at least 40 feet. The highest
cliffs—some approximately 100 feet—on which brush mice are
known to occur in Kansas are along Shoal Creek, Cherokee County.
The brush mouse is found on low bluffs that are parts of higher
systems, but in Cherokee County the mouse was not obtained from
low bluffs separated by even a few miles from the cliff-system along
Shoal Creek. As implied above the brush mouse is adapted for a
scansorial mode of life; but other mice and rats inhabit the rocky
crevices of low bluffs. Whereas the brush mouse is well adapted
for living on high cliffs it seems that the other rodents are better
adapted for life on low cliffs. Sigmodon hispidus was obtained
from the low, limestone cliffs mentioned previously. From most
low bluffs in southeastern Kansas (and on some high bluffs outside
the geographic range of cansensis) Peromyscus leucopus was obtained.
In Cowley County the brush mouse was abundant when
P. leucopus was not and vice versa during this study. Sigmodon
hispidus did not associate with the brush mouse in any area,
although S. hispidus was often trapped in grassy areas adjacent[Pg 105]
to cliffs and on the grassy crests of the hills. Except at the locality
in Cherokee County, the pack rat, Neotoma floridana, was found
in association with the brush mouse. Microtus ochrogaster was the
must abundant rodent in adjacent southwestern Missouri (Jackson,
1907) before Sigmodon thoroughly infiltrated this area and southeastern
Kansas. Activities of other rodents may have confined the
brush mouse ecologically to cliffs. Although the grasslands are a
barrier to further intrusion by the brush mouse into Kansas, one
cannot assume that they alone confined the brush mouse to cliffs.
Such an assumption would not explain its absence on systems of
cliffs similar to and near other systems of cliffs on which it is found
in the non-grassy Ozarkian habitats of Arkansas, as was noticed
by Black (1937). Such an assumption would not indicate why
the size of the cliff-systems is correlated with the absence or presence
of the brush mouse on the northeastern margin of its geographic
range.
Parasites found on P. b. attwateri include three individuals of the
laelapid mite, Haemolaelaps glasgowi. Two of these mites were
removed from a live mouse. Two larval Ixodid ticks, Ixodes
possibly cookei, were removed from the pinnae of the ears of a
specimen of cansensis from the type locality, 4 mi. E Sedan, Chautauqua
County. Four larval Ixodid ticks, Dermacentor possibly
variabilis, were removed from the pinnae of the ears of a live
specimen of cansensis from 3 mi. W Cedar Vale, in Cowley County.
Table 2. Stomach Contents of 38 Brush Mice from Southeastern
Kansas in Winter and Spring.
Localities and number
of stomachs |
Month |
Empty |
Acorn pulp |
Seeds |
2 mi. S Galena |
|
| | |
10 |
|
May, 1959 |
2 | 6 | 2 |
11 |
|
December, 1959 |
1 | 10 | 0 |
3 |
|
March, 1960 |
1 | 2 | 0 |
4 mi. E Sedan |
|
| | |
3 |
|
December, 1959 |
3 | 0 | 0 |
2 |
|
April, 1961 |
1 | 1 | 0 |
3 mi. W Cedar Vale |
|
| | |
6 |
|
December, 1959 |
1 | 3 | 2 |
3 |
|
December, 1960 |
0 |
3[B] |
0 |
Black (1937:195) and Cockrum (1952:180-181) reported stomach[Pg 106]
contents of P. b. attwateri from Cherokee County containing acorn
pulp, seeds, and insects. Analysis of 38 stomachs of the brush
mouse (Table 2) show acorns to be the most commonly used food
in winter and spring. Seed coats were only rarely found, and
insects were absent. Two captive females preferred acorns. Live
beetles and grasshoppers of numerous kinds were decapitated and
their inner parts eaten. Seeds (wheat, corn, and oats) were also
eaten. Inasmuch as acorns appear to be the chief food, it is not
surprising that the brush mouse is usually found on cliffs that support
stands of blackjack oak (Quercus marilandica). Other oaks
are present, but I have no evidence that the brush mouse eats their
acorns. A. Metcalf told me that he observed in December, 1960,
a released brush mouse interrupt its movement toward a hole in a
cliff-face along Cedar Creek, Cowley County, in order to pick up
an acorn (judged to be from the blackjack oak) in daylight. The
mouse carried the acorn into the hole in the cliff. I have observed
that captive brush mice eat acorns of the blackjack oak but not
some other kinds of acorns.
Behavior
The chief differences observed between the brush mouse and
other species of the genus Peromyscus in Kansas can be summarized
as follows: the brush mouse is a superior and more cautious
climber; seldom jumps from high places when under stress; is
capable of finding its way better in partial darkness; has a stronger
preference for acorns; and sometimes buries or hides seeds or
acorns. These are all behavioral adaptations that seem in harmony
with its mode of life.
Buck, Tolman, and Tolman (1925) showed the balancing function
of the tail in Mus musculus. Climbers (for example, squirrels)
often possess long, well-haired tails. It is reasonable to suggest
(as did Hall, 1955:134) that the long, tufted tail is an adaptation
for a scansorial existence. Little observation is necessary to observe
how such a tail is used in balancing. Furthermore, it is used as a
prop when the mouse is climbing a vertical surface. Dalquest
(1955:144) mentioned tree-climbing in P. boylii from San Luis
Potosí, México. It may occur in P. b. attwateri or in P. b. cansensis
also, but there is no evidence as yet to prove it.
The brush mouse can seldom be induced to jump from heights
of two feet or more. Rather it tends to scamper downward or to
remain in place. It often swings itself over an edge, holding to[Pg 107]
it by its hind feet, and sometimes to it lightly with its tail, and
reduces a short jump by almost the length of its body. Such
caution seems to be an adaptation in a mouse that lives as a climber.
Many animals of cavernous habitats have small eyes (see Dobzhansky,
1951:284). Some nocturnal animals (for example, owls)
have large eyes. The brush mouse has large, protuberant eyes;
it lives in the deep crevices and fissures of the cliffs on which it
is found, but it is not strictly a cave-dwelling animal. Perhaps
large eyes aid the brush mouse in performing activities in the
partial darkness of a deep crevice or hole in a cliff. Brush mice
experimentally placed in what appeared to be total darkness fed,
built houses of cotton, and ran and climbed in the usual manner.
On several occasions the captive brush mice hid surplus seeds
and on other occasions hid acorns by burying them and sometimes
by placing them in a small jar. The mice never carried the surplus
food into their house.
Black (1937:195) has claimed that the brush mouse builds a nest
similar to that of the nest of the pack rat, Neotoma floridana. Hall
(1955:134) doubts this to be the case. Dalquest (1953:144) described
a nest of P. boylii from San Luis Potosí as seven inches in
diameter, made of leaves, and found in a hollow tree. Drake
(1958:110) noted that P. b. rowleyi lives in holes and crevices in
rocky bluffs in Durango, México. I have found this to be the case
for P. b. attwateri, as did A. Metcalf (unpublished) for P. b. cansensis.
Nests of sticks and leaves were taken apart by Metcalf,
and all sign indicated only the presence of the pack rat. I have
observed that there are no such houses on the cliffs along Shoal
Creek, Cherokee County, and that no pack rats have been obtained
from there (pack rats have not been reported from Cherokee
County). Blair (1938) found two brush mice (P. b. attwateri) in
the house of a pack rat in Oklahoma. Nests of the brush mice
that occur in Kansas have not been found.
A lactating, pregnant female (KU 81833) of P. b. attwateri,
containing three embryos, was obtained on December 24, 1959, and
shows that this subspecies breeds in winter. Accumulated records
for the subspecies indicates year-round breeding (see Cockrum,
1952:181). Another female obtained on March 27, 1960, was probably
lactating.
Pregnant females of P. b. cansensis (KU 84892, 84895, and
84890) were obtained from the type locality on April 1-2, 1961,[Pg 108]
containing 3, 4, and 5 embryos respectively. This indicates, perhaps,
increased breeding in spring; five was the highest number
of embryos found in brush mice in Kansas.
Population Studies
In the period of my study the populations of brush mice became
smaller, perhaps owing to the severe winter of 1959-1960. In
Cowley County, P. leucopus is now abundant and P. boylii rare
where in December of 1959, the opposite was true. It is also
possible, of course, that trapping has depleted the populations.
Conclusions
1. A new subspecies of brush mouse is named and described
from southern Kansas.
2. The new subspecies has smaller eyes and a shorter tail and
may be more primitive than P. b. attwateri.
3. No significant sexual dimorphism was noted in P. boylii.
4. In Kansas, P. b. attwateri is known only from a single locality;
P. b. cansensis is known from only two localities, both in
Kansas.
5. The cliff-dwelling habit of P. boylii probably isolates populations
from one another.
6. The grasslands constitute a barrier for the brush mouse.
7. In Kansas, P. b. cansensis probably is an older population
than P. b. attwateri.
8. In Kansas the brush mouse is confined to systems of cliffs
that are wooded and that are at least 40 feet in height.
9. The brush mouse may be confined to cliffs in part by activities
of other rodents.
10. The brush mouse commonly associates with the pack rat.
11. Laelapid mites have been found on specimens of P. b.
attwateri.
12. Larval ixodid ticks were found on specimens of P. b. cansensis.
13. Acorns seem to be the chief food of the brush mouse; insects
and seeds are also commonly eaten.
14. The brush mouse is adapted for climbing and probably for a
partly subterranean life.
15. P. b. attwateri breeds in winter, as well as in other parts of
the year.[Pg 109]
16. P. b. cansensis is known to breed in early April.
17. The highest number of embryos obtained from a brush mouse
in Kansas is five.
Acknowledgments
I am indebted to Prof. E. Raymond Hall and to Mr. J. Knox Jones, Jr., for
suggestions and editorial assistance. Prof. R. H. Camin identified the ticks
and mites recorded herein. Mr. A. Metcalf, Mrs. C. F. Long, and Mr. D. L.
Long helped with the field studies and in other ways.
[Pg 110]
Literature Cited
Black, J. D.
1937. Mammals of Kansas. 30th Biennial Report, Kansas State Board of
Agri., 35:116-217.
Blair, W. F.
1938. Ecological relationships of the mammals of the Bird Creek Region,
Northeastern Oklahoma. Amer. Midl. Nat., 20:473-526.
1959. Distributional patterns of vertebrates in the southern U. S. in
relationship to past and present environment. Zoogeography, pp.
463-464 and Fig. 5, January 16.
Buck, C. W., Tolman, N., and Tolman, W.
1925. The tail as a balancing organ in mice. J. Mamm., 6:267-271.
Cockrum, E. L.
1952. Mammals of Kansas. Univ. Kansas Publ., Museum of Nat. Hist.,
7:6, 180-181.
Dalquest, W. W.
1953. Mammals of the Mexican state of San Luis Potosí. Louisiana State
Univ. Studies, Biol. series No. 1, 232 pp.
Dobzhansky, T.
1951. Genetics and the origin of species, 3d ed. New York, Columbia
Univ. Press, x + 364 pp.
Drake, J. D.
1958. The brush mouse, Peromyscus boylii, in southern Durango. Museum
Publ., Michigan State Univ., 1:97-132.
Hall, E. R.
1955. Handbook of mammals of Kansas. Univ. Kansas Mus. Nat. Hist.
Publ. No. 7, 303 pp.
Hay, O. P.
1927. The Pleistocene of the western region of N. America ...
Carnegie Inst. Washington, 346 pp., 12 pls.
Hibbard, C. W.
1955. The Jinglebob interglacial (Sangamon?) fauna from Kansas ...
Museum of Paleo., Univ. Michigan, pp. 179-228, 2 pls.
Jackson, H. H. T.
1907. Notes on some mammals of southwestern Missouri. Proc. Biol.
Soc. Washington, 20:71-74.
Osgood, W. H.
1909. Revision of the mice of the American genus Peromyscus. N.
Amer. Fauna, 28:1-285, April 17.
Ridgway, R.
1912. Color standards and color nomenclature. Washington, D. C., 43
pp., 53 pls.
Transmitted June 30, 1961.
◻
28-8518
Transcriber's Note
The following error is noted,
but left as printed:
Page 105, "the must abundant rodent" should be "the most
abundant rodent"









Comments on "Natural History of the Brush Mouse (Peromyscus boylii) in Kansas With Description of a New Subspecies" :