The Project Gutenberg eBook of Middle American Frogs of the Hyla microcephala Group
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Middle American Frogs of the Hyla microcephala Group
Author: William Edward Duellman
M. J. Fouquette
Release date: December 9, 2010 [eBook #34604]
Most recently updated: January 7, 2021
Language: English
Credits: Produced by Chris Curnow, Tom Cosmas, Joseph Cooper and
the Online Distributed Proofreading Team at
https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK MIDDLE AMERICAN FROGS OF THE HYLA MICROCEPHALA GROUP ***
Typographical Corrections
| Page 533 - | UMZ | => | UMMZ |
| Page 534 - | Diganosis | => | Diagnosis |
| Page 544 - | fontanells | => | fontanelle |
| Page 545 - | prrimary | => | primary |
| Page 547 - | band of of frequencies | => | band of frequencies |
| Page 550 - | ad | => | had |
| Page 551 - | clumbs | => | clumps |
| Page 552 - | acount | => | account |
| Page 557 - | Minchigan | => | Michigan |
[Pg 517]
Museum of Natural History
Middle American Frogs
of the Hyla microcephala Group
BY
WILLIAM E. DUELLMAN AND M. J. FOUQUETTE, JR.
University of Kansas
Lawrence
1968
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Frank B. Cross
Volume 17, No. 12, pp. 517-557, 4 pls. 9 figs.
Published March 20, 1968
University of Kansas
Lawrence, Kansas
PRINTED BY
ROBERT R. (BOB) SANDERS, STATE PRINTER
TOPEKA, KANSAS
1968
31-9419
of the Hyla microcephala Group
WILLIAM E. DUELLMAN AND M. J. FOUQUETTE, JR.
| PAGE | |
| Introduction | 519 |
| Acknowledgments | 520 |
| Materials and Methods | 520 |
| Hyla microcephala Group | 521 |
| Key to Species and Subspecies | 522 |
| Accounts of Species and Subspecies | 523 |
| Cranial Osteology | 540 |
| Analysis of Mating Calls | 544 |
| Life History | 550 |
| Phylogenetic Relationships | 552 |
| Literature Cited | 556 |
The small yellow tree frogs, Hyla microcephala and its relatives,
are among the most frequently heard and commonly collected frogs in
the lowlands of southern México and Central America. The similarities
in size, proportions, and coloration of the different species have
resulted not so much in a multiplicity of specific names, but in
differences of opinion on the application of existing names to the
various taxa. For example, the populations on the Atlantic lowlands
have been known by three names, two of which have been applied to
other taxa. Much of the confusion has been the result of previous
workers' unfamiliarity with the animals in life and unawareness of the
intraspecific geographic variation in the most widespread species.
Independently we undertook studies of these frogs in the field. The
second author worked on the interspecific relationships and isolating
mechanisms in Panamá (Fouquette, 1960b) and later studied the species
in southern México. As part of his survey of the hylids of Middle
America, the first author accumulated field and laboratory data on the
frogs throughout their ranges in México and Central America. The
purpose of this report is to present our findings on the four species
of Middle American frogs that we place in the Hyla microcephala
group. In addition to conventional taxonomic characters, we have
utilized the features of the cranial osteology and have relied heavily
on the data obtained from an analysis of the mating calls.
Furthermore, we have included ecological and distributional data in
our synthesis of interspecific relationships.
Examination of specimens was made possible by the provision of working
space at various institutions or through the loan of specimens. For
their generosity in this manner we are grateful to Richard J. Baldauf,
Charles M. Bogert, James E. Böhlke, Doris M. Cochran, Robert F. Inger,
John M. Legler, Alan E. Leviton, Gerald Raun, Jay M. Savage, Hobart M.
Smith, Robert C. Stebbins, Wilmer W. Tanner, Charles F. Walker, Ernest
E. Williams, and Richard G. Zweifel.
Duellman is especially grateful to Charles W. Myers, Linda Trueb,
Jerome B. Tulecke, and John Wellman for their assistance in the field
and to Linda Trueb for her work on the cranial osteology that is
incorporated in this report. Fouquette is indebted to H. Morgan Smith
and A. C. Collins for assistance in the field, to A. J. Delahoussaye
for assistance in the laboratory, and to W. Frank Blair for use of the
facilities of the sound laboratory at the University of Texas and for
much help in the early stages of this study.
The research reported herein was accomplished mainly through support
by the National Science Foundation (grants NSF G-9827 and GB-1441 to
Duellman and GB-599 to Fouquette). The latter's field work in México
was assisted in part by NSF Grant G-4956 to W. Frank Blair. Some of
the field studies carried out in Panamá by Duellman were supported by
a grant from the National Institutes of Health (NIH GM-12020).
We are grateful to many persons, too numerous to mention, who in
various ways aided our field work in Middle America. We are especially
indebted to Dr. Rodolfo Hernandez Corzo and the late Ing. Luis Macías
Arellano of the Dirección General de la Fauna Silvestre of the Mexican
government for providing permits to collect in México.
For this report, data has been obtained from 2829 preserved frogs, 42
skeletal preparations, 8 lots of tadpoles and young, and 4 lots of
eggs. Much of the material was collected in our independent field
work, which has extended over a period of 11 years.
Measurements were taken in the manner described by Duellman (1956).
Osteological data were obtained from specimens that were cleared in
potassium hydroxide, stained with alizarin red, and stored in
glycerine. Recordings were made by means of Magnemite portable tape
recorders (Amplifier Corp. America). The calls recorded by Fouquette
were analyzed on a Sonagraph (Kay Electric Co.) at the University of
Texas; those recorded by Duellman were analyzed mainly on a Vibralyzer
(Kay Electric Co.) at the University of Kansas and in part on a
Sonagraph at the University of Southwestern Louisiana. Sample calls
were analyzed on all three instruments; the slight differences in
results were found to be less than the error in measurement, so the
data from all sources were combined without correction. The techniques
and terminology of the calls are those defined by Fouquette (1960a,
1960b).
In the accounts of the species we have attempted to give a complete
synonymy. At the end of each species account the localities from which
specimens were examined are listed alphabetically within each state,
province, or department, which in turn are listed alphabetically
within each country. The countries are arranged from north to south.
Localities preceded by an
[Pg 521]
asterisk (*) are not plotted on the accompanying maps due to the
crowding of symbols that would have resulted. Abbreviations for museum
specimens are listed below:
| AMNH | —American Museum of Natural History |
| ANSP | —Academy of Natural Sciences of Philadelphia |
| BMNH | —British Museum (Natural History) |
| BYU | —Brigham Young University |
| CAS | —California Academy of Sciences |
| FMNH | —Field Museum of Natural History |
| KU | —University of Kansas Museum of Natural History |
| MCZ | —Museum of Comparative Zoology |
| MVZ | —Museum of Vertebrate Zoology |
| SU | —Stanford University |
| UIMNH | —University of Illinois Museum of Natural History |
| UMMZ | —University of Michigan Museum of Zoology |
| USC | —University of Southern California |
| USNM | —United States National Museum |
| UU | —University of Utah |
| TCWC | —Texas Cooperative Wildlife Collection |
| TNHM | —Texas Natural History Museum |
Definition.—Small hylids attaining a maximum snout-vent length of
27 mm. in males and 32 mm. in females; dorsum yellowish tan with brown
markings; thighs uniformly yellow, vocal sac in breeding males yellow;
snout truncate in lateral profile; tympanum distinct, usually slightly
smaller than one-half diameter of eye; vocal sac single, median,
subgular; fingers about one-third webbed; toes webbed nearly to bases
of discs, except only to middle of antepenultimate or base of
penultimate phalanx of fourth toe; tarsal fold weak; inner metatarsal
tubercle low, flat, elliptical; axillary membrane present; pupil
horizontally elliptical; palpebral membrane unmarked; cranial elements
reduced in ossification; sphenethmoid small, short; frontoparietal
fontanelle large; tegmen tympani not extensive; quadratojugal greatly
reduced; anterior arm of squamosal extending only about one-fourth
distance to maxillary; posterior arm of squamosal not having bony
connection with proötic; nasals lacking maxillary processes; medial
ramus of pterygoid not having bony attachment to proötic; maxillary,
premaxilary, and prevomerine teeth present; palatine and parasphenoid
teeth absent; Mentomeckelians ossified; tadpoles having xiphicercal
tails with deep caudal fins and terminal mouth lacking teeth; mating
call consisting of one primary note followed by a series of shorter
secondary notes; haploid number of chromosomes, 15 (known only in H.
microcephala and H. phlebodes.)
Content.—As recognized here the Hyla microcephala group contains
four species, one having two subspecies. An alphabetical list of the
specific and subspecific names that we consider to be applicable to
the Hyla microcephala group are listed below.
| Names Proposed | Valid Names |
| Hyla cherrei Cope, 1894 | ? = H. m. microcephala |
| Hyla microcephala Cope, 1886 | = H. m. microcephala |
| Hyla microcephala Boulenger, 1898 (nec Cope, 1886) | = H. microcephala underwoodi |
| Hyla microcephala martini Smith, 1951 | = H. microcephala underwoodi |
| Hyla microcephala sartori Smith, 1951 | = H. sartori |
| Hyla phlebodes Stejneger, 1906 | = H. phlebodes |
| Hyla robertmertensi Taylor, 1937 | = H. robertmertensi |
| Hyla underwoodi Boulenger, 1899 | = H. microcephala underwoodi |
[Pg 522]
Discussion.—The color pattern is the most useful character in
distinguishing the species of the Hyla microcephala group from one
another. Except in Hyla microcephala, little geographic variation in
color pattern is noticeable. The features of color pattern that are
helpful in identifying the species are: 1) presence or absence of
lateral dark brown stripe; 2) longitudinal extent and width of lateral
stripe, if present; 3) presence or absence of a narrow white line just
dorsal to the lateral dark stripe; 4) presence or absence of an
interorbital dark mark; 5) the arrangement of dark markings on the
back, either as longitudinal lines or series of dashes, or in the form
of various kinds of transverse markings; 6) presence of dark flecks,
longitudinal line, or transverse marks on shanks.
Few consistent differences in measurements and proportions exist among
the species (Table 1). The most obvious morphological difference is
that the head is noticeably narrower in H. robertmertensi than in
the other species. Hyla phlebodes is the smallest species; adult
males attain snout-vent lengths of only 23.6 mm. The body is slender
in H. microcephala and robertmertensi, slightly wider in
phlebodes, and noticeably broader in sartori.
Distribution.—The composite range of the Middle American frogs of
the Hyla microcephala group includes the lowlands of southern México
and Central America, in some places to elevations of 1200 meters,
southeastward from southern Jalisco and southern Veracruz, excluding
arid regions (northern Yucatán Peninsula, Balsas-Tepalcatepec Basin,
Plains of Tehuantepec, Grijalva Valley, Salamá Basin, and upper
Motagua Valley) to the Pacific lowlands and the Cauca and Magdalena
valleys in Colombia.
| 1. | Lateral dark stripe, bordered above by narrow white line, extending from snout at least to sacral region2 |
| Lateral dark stripe, if present, not extending posteriorly to sacral region and not bordered above by narrow white line4 | |
| 2. | Lateral dark stripe continuous to groin; dark flecks or longitudinal line on shanks; interorbital dark bar absent; dorsal pattern usually consisting of pair of longitudinal dark lines or series of dashes3 |
| Lateral dark stripe usually extending only to sacral region; dark transverse bars on shanks; interorbital bar usually present; dorsal pattern usually consisting of interconnecting dark lines, sometimes forming transverse marksH. microcephala underwoodi | |
| 3. | Lateral dark stripe narrow, covering only upper edge of tympanum; dorsal longitudinal stripes continuous, extending to ventH. microcephala microcephala |
| Lateral dark stripe wide, encompassing entire tympanum; dorsal markings consisting of longitudinal series of flecks or dashes, or of two lines, usually not extending to vent H. robertmertensi | |
[Pg 523] | |
| 4. | Lateral dark stripe indistinct, present only above tympanum and insertion of arm; dorsal markings consisting of narrow lines and dashes, sometimes interconnected; transverse bars on shanks narrow relative to interspacesH. phlebodes |
| Lateral dark stripe absent; dorsal markings consisting of two broad chevron-shaped marks; transverse bars on shanks wide relative to interspacesH. sartori | |
Diagnosis.—Lateral dark stripe narrow, covering only upper edge of
tympanum, bordered above by narrow white stripe; dorsal pattern
consisting of pair of longitudinal brown lines and no interorbital bar
(eastern populations), or of irregular dark markings forming an X- or
)(-shaped mark in scapular region and an interorbital bar (western
populations).
Content.—The populations inhabiting the Pacific lowlands of
southeastern Costa Rica eastward to Colombia are recognized herein as
Hyla microcephala microcephala Cope; the populations in western
Costa Rica northward to México are assigned to Hyla microcephala
underwoodi Boulenger.
Distribution.—Southern Veracruz and northern Oaxaca southeastward
through the Atlantic lowlands of Central America to north-central
Nicaragua, thence southeastward on the Pacific lowlands to eastern
Panamá, and thence into the Cauca and Magdalena valleys (Caribbean
drainage) of Colombia (Fig. 1).
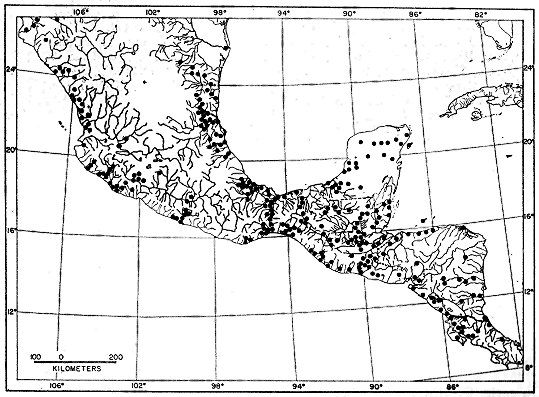
Fig. 1. Map showing locality records for Hyla microcephala.
Mean and Standard Error of Mean Below Observed Range.)
| Locality | N | Snout-vent length (S-V L) | Tibia length S-V L | Foot length S-V L | Head length S-V L | Head width S-V L | Tympanum Eye |
H. m. microcephala | |||||||
| Panamá: Canal Zone | 25 | 21.5-24.1 | 50.2-56.0 | 40.9-46.6 | 28.5-32.8 | 28.1-30.9 | 44.0-54.1 |
| 22.8±0.20 | 52.9±0.37 | 43.5±0.28 | 31.0±0.22 | 29.4±0.11 | 49.0±0.55 | ||
| Costa Rica: Golfito | 25 | 18.5-24.5 | 49.1-54.4 | 41.8-48.0 | 30.2-35.5 | 29.0-32.7 | 40.0-57.8 |
| 22.4±0.27 | 51.6±0.26 | 45.1±0.32 | 33.1±0.25 | 30.8±0.16 | 48.4±1.10 | ||
H. m. underwoodi | |||||||
| Nicaragua: La Cumplida | 25 | 23.0-25.6 | 51.0-55.7 | 41.3-46.5 | 29.7-33.5 | 28.9-31.8 | 42.3-60.0 |
| 24.1±0.19 | 52.9±0.25 | 43.7±0.25 | 31.6±0.19 | 30.4±0.17 | 49.3±0.97 | ||
| Guatemala: Finca Chamá | 25 | 21.8-25.0 | 51.0-57.2 | 41.2-47.8 | 30.8-35.3 | 29.6-33.6 | 37.5-56.4 |
| 23.5±0.16 | 54.3±0.39 | 44.4±0.30 | 33.0±0.16 | 31.3±0.36 | 45.2±0.89 | ||
| Tabasco: Teapa | 25 | 22.7-25.8 | 48.0-54.5 | 40.7-46.8 | 29.5-33.0 | 28.7-31.8 | 40.7-53.8 |
| 24.3±0.14 | 51.5±0.29 | 43.3±0.25 | 31.7±0.17 | 30.3±0.16 | 45.5±0.38 | ||
| Oaxaca: Donají-Sarabia | 25 | 22.1-25.9 | 49.8-55.6 | 40.5-46.6 | 30.4-34.8 | 28.9-32.6 | 37.0-54.1 |
| 23.8±0.19 | 52.8±0.33 | 43.4±0.27 | 32.8±0.19 | 30.8±0.17 | 45.1±0.76 | ||
| Veracruz: Alvarado | 25 | 21.9-25.4 | 49.6-54.4 | 40.7-47.5 | 29.9-33.8 | 29.1-32.9 | 40.7-53.8 |
| 24.1±0.17 | 51.1±0.28 | 42.6±0.34 | 31.4±0.18 | 30.5±0.17 | 46.6±0.65 | ||
[Pg 525] H. robertmertensi | |||||||
| Guatemala: La Trinidad | 21 | 21.8-24.6 | 47.1-52.8 | 40.9-51.3 | 30.0-33.3 | 27.3-29.8 | 44.4-50.0 |
| 23.4±0.15 | 49.9±0.34 | 43.5±0.17 | 31.3±0.20 | 28.5±0.23 | 47.4±0.46 | ||
| Chiapas: Acacoyagua | 25 | 21.4-25.7 | 47.8-52.4 | 41.7-46.3 | 29.1-32.7 | 26.0-30.3 | 42.8-53.8 |
| 24.1±0.20 | 50.4±0.45 | 43.9±0.23 | 31.2±0.29 | 28.1±0.20 | 46.5±0.50 | ||
| Oaxaca: Tapanatepec | 25 | 22.4-26.4 | 44.1-48.3 | 39.1-44.5 | 26.1-30.4 | 25.4-28.1 | 45.8-58.3 |
| 24.7±0.18 | 46.4±0.23 | 41.7±0.23 | 28.4±0.16 | 26.8±0.14 | 52.9±0.77 | ||
H. phlebodes | |||||||
| Panamá: Canal Zone | 25 | 19.6-23.2 | 49.1-56.9 | 41.9-47.1 | 33.6-37.4 | 32.3-36.0 | 37.9-46.4 |
| 22.2±0.16 | 52.8±0.35 | 45.4±0.26 | 34.8±0.18 | 33.8±0.18 | 41.6±0.49 | ||
| Costa Rica: Turrialba | 25 | 19.7-23.6 | 47.4-55.7 | 38.1-46.4 | 32.6-35.9 | 30.5-35.0 | 35.7-48.2 |
| 22.0±0.18 | 51.1±0.35 | 42.8±0.38 | 34.1±0.16 | 32.9±0.17 | 40.1±0.53 | ||
H. sartori | |||||||
| Guerrero: Tierra Colorada | 25 | 23.7-26.0 | 47.2-51.4 | 42.4-47.8 | 29.4-31.8 | 28.9-31.0 | 42.3-52.0 |
| 24.8±0.13 | 49.6±0.23 | 45.2±0.27 | 30.6±0.13 | 30.0±0.12 | 47.4±0.59 | ||
[Pg 526]
Hyla microcephala Cope, Proc. Amer. Philos. Soc., 23:281, February
11, 1886 [Syntypes.—USNM 13473 (2 specimens, now lost) from Chiriquí,
Panamá; Mr. MacNeil collector]; Bull. U.S. Natl. Mus., 32:14, 1887.
Günther, Biologia-Centrali Americana, Reptilia and Batrachia, p. 265,
June, 1901. Dunn, Occas. Papers Boston Soc. Nat. Hist., 5:413, October
10, 1931; Occas. Papers Boston Soc. Nat. Hist., 8:72, June 7, 1933.
Stebbins and Hendrickson, Univ. California Publ. Zool., 56:524,
February 17, 1959. Fouquette, Evolution, 14:484, December 16, 1960.
Busack, Copeia, 2:371, June 21, 1966.
? Hyla cherrei Cope, Proc. Acad. Nat. Sci. Philadelphia, 1894, p.
195, 1894 [Holotype.—location unknown, apparently lost;
type-locality: "Alajuela, Costa Rica;" R. Alfaro collector]. Günther,
Biologia Centrali-Americana: Reptilia and Batrachia, p. 264, June,
1901. Taylor, Univ. Kansas Sci. Bull., 35:846, July 1, 1952.
Hyla underwoodi, Ruthven, Misc. Publ. Mus. Zool., Univ. Michigan,
8:55, September 15, 1922. Barbour, Proc. New England Zool. Club,
10:31, March 2, 1928.
Hyla microcephala microcephala, Smith, Herpetologica, 7:185,
December 31, 1951. Taylor, Univ. Kansas Sci. Bull., 39:23, November
18, 1958.
Diagnosis.—Brown lateral stripe narrow, extending from nostril
along canthus, along upper edge of tympanum to groin, bordered above
by narrow white line; pair of dark brown longitudinal lines on dorsum
extending to vent; shanks having dark longitudinal line or flecks, no
transverse bars; interorbital dark mark lacking.
Description and Variation.—The color pattern is nearly constant. Of
103 males from the Canal Zone, all lack an interorbital dark bar, and
all have a dark longitudinal line on the dorsal surface of the shank
and a narrow lateral dark stripe, bordered above by a narrow white
line, extending to the groin. The longitudinal dark lines on the
dorsum are continuous to the groin in 95 specimens and fragmented in
two specimens. In two others the lines converge and fuse in the
scapular region, and in four specimens auxiliary, fragmented lines are
present dorsolaterally.
In all specimens from southeastern Costa Rica (Golfito, Palmar Sur,
and Villa Neilly) the pattern is constant, except that in about 10 per
cent of the specimens the longitudinal line on the dorsal surface of
the shank is replaced by a row of brown flecks.
Of the limited number of Colombian specimens examined, all are
patterned normally, except three from Sautata, Chocó, three from
Curumani, and three from Arcataca, Magdalena, which have flecks on the
dorsal surfaces of the shanks, and one from Espinal, Tolima, which has
no markings on the shanks.
When active at night most individuals are pale yellowish tan dorsally;
the white dorsolateral line is noticeable, but the brown lateral
stripe, dorsal brown lines, and lines on shanks are so pale that often
they are barely discernible. By day the dorsum changes to tan or pale
reddish brown; the stripes are dark brown, and the dorsolateral stripe
that is white at night becomes creamy yellow (Pl. 13). Small brown
flecks are present on the dorsum of most individuals. The venter
always is white, and the iris is pale bronze with a brown tint
immediately anterior and posterior to the pupil. In breeding males the
vocal sac is pale yellow.
Tadpoles.—Tadpoles of this species have been found in weed-choked
ponds in eastern Panamá Province. The following description is based
on KU 104097, a specimen in developmental stage 34 (Gosner, 1960).
[Pg 527]
Total length, 20.5 mm.; body length, 8.2 mm.; body slightly wider
than deep; snout pointed; nostrils large, situated dorsally, much
closer to snout than eyes, directed anteriorly; eyes moderately
small, situated dorsolaterally and directed laterally; spiracle
sinistral, located just posteroventral to eye; anal tube dextral.
Tail xiphicercal; caudal musculature moderately deep, becoming
slender posteriorly, extending beyond caudal fin; fins deepest at
about one-third distance from body to tip of tail; dorsal fin
extending onto body, deeper than deepest part of caudal
musculature; ventral fin slightly shallower than musculature.
Mouth small, terminal, lacking teeth and fringing papillae, but
having finely serrate beaks. In preservative, top of head pale
brown; dark stripe from tip of snout through eye to posterior edge
of body, narrowing to thin line on proximal one-fourth of tail;
venter white; tail creamy tan with fine black flecks most numerous
posteriorly; posterior two-thirds of fins edged with black. In
life, top of head yellowish tan; lateral stripe brown; belly
white; anterior half of tail lacking pigment; posterior half deep
orange; iris pale bronze (Pl. 15).
Remarks.—Evidence for intergradation of Hyla microcephala with
H. underwoodi is provided by four specimens [USC 818 (2), 6081-2]
from 6.1 kilometers northeast of the mouth of the Río Tarcoles, and
nine specimens [USC 8254 (2), 8255, 8256 (4), 8258 (2)] from Parrita,
both in Puntarenas Province, Costa Rica. These localities lie about
two-thirds the distance from the northwesternmost locality for H.
m. microcephala (Palmar Sur) to the southeasternmost locality for
H. m. underwoodi (Barranca). Although in most aspects of coloration
the frogs are more nearly like H. m. underwoodi than H. m.
microcephala, some specimens have longitudinal lines on their shanks,
such as are characteristic of H. m. microcephala. The dorsal pattern
varies from nearly complete longitudinal lines to broken lines, fused
into an X-shaped scapular mark or not.
As noted by Rivero (1961:135), Hyla microcephala seems to be closely
related to Hyla misera Werner, a species having a wide distribution
east of the Andes in South America. Despite the similarity in color
pattern, size, and structure, we are reluctant to place the two taxa
in the same species until data on coloration in life, mating calls,
and life history are available for Hyla misera and compared with
those of Hyla microcephala.
The status of Cope's Hyla cherrei is questionable. Since the type,
the only specimen ever referred to the species, apparently is lost,
the only extant information regarding the taxon is contained in the
original description (Cope, 1894). There the species was characterized
as having a narrow dorsolateral white stripe and lacking pigment on
the upper arms and thighs. These characteristics of the color pattern
combined with the statements "vomerine teeth few, opposite the middle
of the very large choanae" and
[Pg 528]
"tympanic drum distinct, one half the area of eye" serve to
distinguish H. cherrei from all other Costa Rican hylids, except H.
m. microcephala and H. m. underwoodi. No statements in the type
description will definitely associate cherrei with one or the other
of these subspecies. Since it seems obvious that H. cherrei can be
associated with H. microcephala, we prefer to place the name in the
synonymy of the nominate subspecies, thereby preserving the commonly
used name H. underwoodi (Boulenger, 1899) as a subspecies of H.
microcephala.
Distribution.—Hyla microcephala microcephala inhabits coastal
lowlands from the area of Golfo Dulce (apparently absent from the
Osa Peninsula) in southeastern Costa Rica eastward in Panamá,
including the Azuero Peninsula to northern Colombia and thence
southward in the valleys of the Río Cauca and Río Magdalena in
Colombia (Fig. 1). Except for the central area of the Canal Zone
the subspecies is unknown from the Caribbean drainage in Central
America, but in Colombia the subspecies occurs only in the
Caribbean drainage. In Central America this frog occurs mostly on
the coastal lowlands; the highest recorded elevation is 560 meters
at El Valle, Coclé, Panamá. Throughout most of its range Hyla
microcephala microcephala occurs in disturbed habitats—cut-over
forests, secondary growth, and pastureland. It does not seem to be
an inhabitant of either primary forest or of Curatella-savanna.
Specimens examined.—522, as follows: Costa Rica: Puntarenas:
Golfito, KU 32172-207; 3 km. E Golfito, KU 86399, USC 2757-8;
Palmar Sur, KU 64591-608, USC 2650 (14), UU 3907-32; *1.5-2.5 km.
ESE Palmar Sur, KU 68293-7 (skeletons), 93957-62; Parrita, USC
8254 (2), 8255, 8256 (4), 8258 (2) [intergrades with H. m.
underwoodi]; 3 km. NW Piedras Blancas, KU 103689; 6.1 km. NE
mouth of Río Tárcoles, USC 818 (2), 6081-2 [intergrades with H.
m. underwoodi]; Villa Neilly, USC 2651; *1-5 km. WNW Villa
Neilly, USC 6182-4, 8003 (4), 8031 (3), 8032; *10.5 km. WNW Villa
Neilly, KU 64609-27, 68398 (eggs).
Panama: Canal Zone: Albrook Air Base, TNHC 23389, 23497; Balboa,
ANSP 19555-6; *Fort Clayton, UIMNH 42008-12; *2.8 km. SW Fort
Kobbe, KU 96015-25; *Frijoles, MCZ 19208; *Bamboa, MCZ 21507; *8.3
km. N Gatún Locks, TNHC 23441; *Juan Diaz, MCZ 13747; *Juan Mina,
AMNH 55436-7, ANSP 21811-2, UMMZ 126734, 126735 (6), UU 3900-6;
*8-14 km. N Miraflores Locks, TNHC 23374-88, 23390-409, 23411-38,
23440, 23442-60, 23462-76; 23478-83, 23492, 23555-60, 23562-76;
*Río Chagres, AMNH 55430, 55439; *Río Cocolí, 3.5 km. N Miraflores
Locks, TNHC 23410; *Summit, ANSP 23365-71, FMNH 22966-9, KU
97783-87. Chiriqui: 5.5 km. E Concepción, AMNH 69772; *14.4 km. E
Concepción, AMNH 69773-8; 2 km. S David, AMNH 69779; *Progreso,
UMMZ 58252, 58253 (2), 58254, 58436; Río Gariché, 8.3 km. ESE Paso
Canoas, KU 103065-8. Coclé: 1 km. SE El Caño, KU 103042-51; El
Valle de Antón, AMNH 59614-18 (10), 69785, ANSP 23502-5, KU
77201-14, MVZ 66578-83, UIMNH 46532. Colón: Cement Plant,
Transisthmian Highway, FMNH 60394-5. Darién: El Real, KU 80454-5,
103052-64, UMMZ 125036 (10), USNM 140567-8; Río Canclon at Río
Chucunaque, UMMZ 125035; *Río Chucunaque, near Yavisa, AMNH 59523.
Los Santos: Tonosí, KU 101606-9. Panamá: 5 km. S Bejuco, AMNH
69782; 3 km. W Chepo, KU 77172-4, 104097-8 (tadpoles); *6 km. WSW
Chepo, KU 77175; *Chico, Río La Jagua, USNM 129070; *La Joya,
Cacora, ANSP 25129-33; Madden Dam, FMNH 67819; Nueva Gorgona, AMNH
69780-1; *1.6 km. W Nueva Gorgona, AMNH 69783-4; 1.5 km. W Pacora,
77176-200; *Río La Laja, near Chamé, ANSP 21845; *Río Tapia, MCZ
10048; *Tapia, AMNH 18930, 18950, 18952-3; *18 km. E Tocumen, MVZ
78662.
[Pg 529]
Colombia: Chocó: Sautatá, Atrato, FMNH 74918 (2), 74919.
Magdalena: Aracataca, ANSP 19755-7; Curumani, MCZ 21465-74, UIMNH
28855; UMMZ 90168, USNM 118247; El Banco, Río Magdalena, ANSP
25061; Fundación, UMMZ 48281-2. Tolima: Espinal, MCZ 15068;
Mariquita, FMNH 81822-3. Valle: Sevilla, MCZ 13751-3.
Hyla microcephala Boulenger, Proc. Zool. Soc. London, p. 481,
October 1, 1898 [Syntypes.—BMNH 94. 11. 1532-33 from Bebedero,
Guanacaste Province, Costa Rica; C. F. Underwood collector] (not
Hyla microcephala Cope, Proc. Amer. Philos. Soc., 23:281,
February 11, 1886, from Chiriquí, Panamá).
Hyla underwoodi Boulenger, Ann. Mag. Nat. Hist., ser. 7, 3:277,
April, 1899 (substitute name for Hyla microcephala Boulenger,
preoccupied). Günther, Biologia-Centrali Americana, Reptilia and
Batrachia, p. 278, September, 1901. Dunn and Emlen, Proc. Acad.
Nat. Sci. Philadelphia, 84:25, March 22, 1932. Stuart, Misc. Publ.
Mus. Zool., Univ. Michigan, 29:39, October 1, 1935. Taylor, Proc.
Biol. Soc. Washington, 50:44, April 21, 1937. Stuart, Occas.
Papers Mus. Zool., Univ. Michigan, 471:15, May 17, 1943. Taylor
and Smith, Proc. U. S. Natl. Mus., 95:586, June 30, 1945. Stuart,
Misc. Publ. Mus. Zool., Univ. Michigan, 69:35, June 12, 1948.
Smith and Taylor, Bull. U. S. Natl. Mus., 194:85, June 17, 1948;
Univ. Kansas Sci. Bull., 33:316, March 20, 1950. Stuart, Contr.
Lab. Vert. Biol., Univ. Michigan, 45:48, May, 1950. Taylor, Univ.
Kansas Sci. Bull., 35:891, July 1, 1952; Univ. Kansas Sci. Bull.,
39:25, November 18, 1958.
Hyla phlebodes, Cole and Barbour, Bull. Mus. Comp. Zool.,
50:154, November, 1906. Kellogg, Bull. U. S. Natl. Mus., 160:172,
March 31, 1932.
Hyla microcephala martini Smith, Herpetologica, 7:187, December
31, 1951 [Holotype.—UIMNH 20965 from Encarnacion, Campeche,
México; H. M. Smith collector]. Stuart, Contr. Lab. Vert. Biol.,
Univ. Michigan, 68:46, November, 1954. Fugler and Webb,
Herpetologica, 13:105, July 10, 1957. Stuart, Contr. Lab. Vert.
Biol., Univ. Michigan, 75:17, June, 1958. Neill and Allen, Publ.
Research Div., Ross Allen's Reptile Inst., 2:26, November 10, 1959.
Duellman, Univ. Kansas Publ., Mus. Nat. Hist., 13:62, August 16,
1960. Stuart, Herpetologica, 17:74, July 11, 1961. Hensley and
Smith, Herpetologica, 18:70, April 9, 1962. Stuart, Misc. Publ.
Mus. Zool., Univ. Michigan, 122:36, April 2, 1963. Holman and
Birkenholz, Herpetologica, 19:144, July 3, 1963. Duellman, Univ.
Kansas Publ., Mus. Nat. Hist., 15:225, October 4, 1963; Univ.
Kansas Publ., Mus. Nat. Hist., 15:588, June 22, 1965.
Hyla microcephala underwoodi, Smith, Herpetologica, 7:188,
December 31, 1951.
Diagnosis.—Brown lateral stripe narrow, extending to groin or
only to sacral region, bordered above by narrow white line; dorsal
pattern bold, consisting of X- or )(-shaped mark in scapular
region or pair of interconnected dark lines on back; interorbital
dark mark usually present; shanks usually having dark transverse
bars.
Description and Variation.—The dorsal color pattern is highly
variable. The various permutations of the X-shaped scapular mark
and dark sacral marks differ proportionately in different samples.
The variation in color pattern in 12 samples is summarized in
Table 2. In samples from the southern part of the range (southern
Nicaragua and Guanacaste Province, Costa Rica) more (40-93%)
individuals have the lateral stripes extending to the groin than
in northern samples (0-42%) from southern México and Guatemala.
Likewise, the percentage of specimens lacking bars on the shanks
and a dark interorbital bar is higher in the Costa Rican samples
than elsewhere in the range. The X- or )(-shaped scapular markings
and /\- or / \-shaped sacral markings are most prevalent in northern samples,
whereas to the south the dorsal markings are more commonly arranged in a
pattern of paired lines, which usually are discontinuous and usually extend
posteriorly only to the sacral region. Thus, the color pattern in H. m.
underwoodi in the southern part of its range shows trends towards the
pattern characteristic of H. m. microcephala. Intergrades between
these two subspecies have been discussed in the account of the
nominate subspecies.
[Pg 530]
| Population | N | Shanks | Interorbital bar | Dorsolateral stripe | Scapular markings | Sacral markings | ||||||||||||
| Bars | Flecks | Present | Absent | Groin | Sacrum | X | )( | ][ | Other | /\ | / \ | Other | ||||||
| Oaxaca: Donají-Sarabia | 27 | 22 | 5 | 27 | 0 | 0 | 27 | 23 | 4 | 0 | 0 | 7 | 6 | 14 | ||||
| Tabasco: Teapa-Villahermosa | 55 | 46 | 9 | 55 | 0 | 0 | 55 | 53 | 2 | 0 | 0 | 19 | 11 | 23 | ||||
| Guatemala: La Libertad | 51 | 51 | 0 | 51 | 0 | 17 | 34 | 45 | 6 | 0 | 0 | 16 | 14 | 21 | ||||
| Guatemala: Finca Chamá | 32 | 32 | 0 | 32 | 0 | 0 | 32 | 32 | 0 | 0 | 0 | 26 | 2 | 4 | ||||
| Guatemala: Puerto Barrios | 31 | 31 | 0 | 31 | 0 | 14 | 17 | 23 | 0 | 4 | 4 | 6 | 4 | 21 | ||||
| Honduras: Lago Yojoa | 13 | 13 | 0 | 13 | 0 | 9 | 4 | 3 | 2 | 3 | 5 | 2 | 1 | 10 | ||||
| Nicaragua: La Cumplida | 56 | 44 | 12 | 54 | 2 | 13 | 43 | 11 | 35 | 8 | 2 | 0 | 19 | 37 | ||||
| Nicaragua: Tipitapa | 10 | 10 | 0 | 10 | 0 | 8 | 2 | 0 | 5 | 3 | 2 | 0 | 3 | 7 | ||||
| Nicaragua: Santo Thomás | 10 | 10 | 0 | 10 | 0 | 8 | 2 | 3 | 0 | 7 | 0 | 0 | 5 | 5 | ||||
| Costa Rica: Tenorio-Tilarán | 12 | 0 | 12 | 6 | 6 | 7 | 5 | 0 | 0 | 12 | 0 | 0 | 0 | 12 | ||||
| Costa Rica: Las Cañas-Liberia | 38 | 21[A] | 15 | 34 | 4 | 25 | 13 | 0 | 11 | 19 | 8 | 0 | 0 | 38 | ||||
| Costa Rica: Esparta | 32 | 26 | 6 | 29 | 3 | 30 | 2 | 0 | 0 | 14 | 18 | 0 | 0 | 32 | ||||
[A] Longitudinal stripes present in two specimens.
[Pg 531]
When this frog is active at night its dorsum is pale yellow; faint
flecks are present in some individuals. The white dorsolateral line
usually is evident in the tympanic region, but in many individuals a
dorsal pattern of lines and other marks is not evident. By day the
dorsum changes to yellowish tan or pale brown with dark brown or
reddish brown markings (Pl. 13). The venter is white, and the vocal
sac in breeding males is yellow. The iris is pale bronze with a brown
tint anterior and posterior to the pupil.
Remarks.—Hyla microcephala underwoodi has had a confused
nomenclatural history. The taxon was first named Hyla microcephala
by Boulenger (1898); this name was preoccupied by Hyla microcephala
Cope (1886). Cole and Barbour (1906) and Kellogg (1932) used the name
Hyla phlebodes Stejneger (1906) for specimens of this frog from
México. Dunn (1931, 1933, 1934) applied the name Hyla underwoodi to
Panamanian specimens that we identify as Hyla phlebodes. Smith
(1951) named Hyla microcephala martini from southern México and
Guatemala and considered the northern populations to represent a
subspecies distinct from the Costa Rican Hyla microcephala
underwoodi, despite the fact the Stuart (1935:39) stated that
comparisons of specimens from El Petén, Guatemala, with the holotype
of Hyla underwoodi showed only trivial differences.
Much of the confusion regarding the name Hyla underwoodi stems from
the illustration given by Boulenger (1898:pl. 39, fig. 3) and
reproduced by Taylor (1952:892), which shows a frog having a unicolor
dorsum, dorsolateral white lines, and dark flanks. This pattern is in
marked contrast to the pattern seen in most preserved specimens, which
have the dorsum variously marked by dark brown lines or irregular
marks. Smith (1951:185), in his description of Hyla microcephala
martini from southern México, considered H. underwoodi to be a
subspecies of H. microcephala that lacked dorsal dark markings.
Data accumulated in 1961 through field studies by the senior
author at the type locality, Bebedero, and other localities in Guanacaste
and Puntarenas provinces in Costa Rica provide a reasonable
explanation of the differences in color pattern. As noted in the
preceding description of this subspecies, at night the dorsal markings
[Pg 532]
are not evident in many living individuals, whereas by day
the dorsal markings are prominent. Most collectors prepare their
specimens by day; consequently the majority of specimens have a
pronounced dorsal pattern. Of the frogs collected in Costa Rica
in 1961, some specimens were preserved at night; others from the
same series were preserved by day. The differences are striking.
In those preserved at night, dorsal markings are faint, if present at
all. Some specimens closely match the figure given by Boulenger
(1898).
It is extremely doubtful if the frog described and illustrated by
Boulenger could be associated with either Hyla phlebodes or H.
microcephala microcephala. Individuals of the former species lack
a dorsolateral white line and always have some dorsal markings
evident at night; furthermore, H. phlebodes is not known to occur
on the Pacific lowlands. Hyla microcephala microcephala occurs
farther southeast. Since there is no reason to doubt the type locality
of H. underwoodi, since specimens from the area around the type
locality that have been preserved at night are like the holotype in
pattern, and since the characteristics of the populations of the frogs
in Guanacaste are the same as, or gradually blend into those of,
populations in northern Central America and southern México, the
frogs from throughout the entire range can be referred to one taxon,
the earliest name for which is Hyla underwoodi Boulenger, which
herein is considered to be a subspecies of H. microcephala Cope.
Distribution.—Hyla microcephala underwoodi inhabits the
Atlantic slopes and lowlands from southern Veracruz and extreme
northern Oaxaca eastward across the base of the Yucatan Peninsula
(possibly the species is extant in the northern part of the
peninsula) to British Honduras and thence southeastward through
the Caribbean lowlands and interior valleys in Honduras to central
Nicaragua, where it apparently avoids the forested Caribbean
lowlands and the dry Pacific lowlands of northwestern Nicaragua,
but in the vicinity of Managua invades the Pacific lowlands and
continues southward into northwestern Costa Rica as far as the
Puntarenas Peninsula (Fig. 1). In México and Guatemala the species
has not been taken at elevations of more than 350 meters, whereas
farther south it occurs at higher elevations—780 meters at
Silencio, Costa Rica, 830 meters on Montaña de Guaimaca, Honduras,
960 meters at Finca Tepeyac, Nicaragua, and 1200 meters at Finca
Venecia, Nicaragua.
Specimens examined.—1270, as follows: Mexico:
Campeche: Balchacaj, FMNH 100406, UIMNH 20944-6;
Encarnación, FMNH 27069-70, 75784, MCZ 28360, 29637, UIMNH
20948-58, 20965, USNM 134264-5; Escárcega, UMMZ 122999; *7.5 km. W
Escárcega, KU 71229-43; Laguna Alvarado, 65 km. S Xpujil, KU
75084-9; Pacaitún, Río Candelaria, FMNH 83118-20; *Tres Brazos,
FMNH 113101-22, UIMNH 20947; 10 km. W Xpujil, KU 75082-3. Chiapas:
Palenque, UIMNH 47984, 49139-50, USNM 114973-8. Oaxaca: *5 km. N
Chiltepec, KU 87015-23; 3 km. N Donají UMMZ 115249 (9); *3.7 km. N
Donají, UMMZ 115250 (5); *43 km. N Matías Romero, UIMNH 42550-68;
*3.5 km. N Palomares, TNHC 25185, 25321-31, 25341-68; 4.6 km.
[Pg 533]
N Sarabia, UMMZ 115247 (2); *6.1 km. N Sarabia, UMMZ 115248 (11), *3
km. N Tolocita, KU 39655; Tuxtepec, KU 87024-40. Tabasco: 24 km. N
Frontera, MCZ 35665-70; 0.8 km. E Río Tonolá, TNHC 25189; Teapa, UMMZ
119218 (4); *2.7 km. N Teapa, UMMZ 119216 (4); *10 km. N Teapa, UMMZ
119217 (6); *11.5 km. N Teapa, UMMZ 119219; *15.2 km. N Teapa, UMMZ
119220 (4); *17.6 km. N Teapa, UMMZ 119221 (12), 3.3 km. S
Villahermosa, UMMZ 119215 (12), *17.6 km. S Villahermosa, UMMZ 119214
(12). Veracruz: 2.1 km. N Acayucan, UIMNH 42547-9; *6.4 km. NW
Acayucan, UMMZ 115254 (14); 1.6 km. ESE Alvarado, UMMZ 115258 (39);
*2.4 km. ESE Alvarado, UMMZ 115251 (2); *4.5 km. S Aquilera,
UMMZ115252 (21); *8 km. SW Coatzacoalcos, UMMZ 119213 (10);
2.2 km. E Cosoleacaque, UMMZ 119222 (26); 10 km. SE Hueyapan, UMMZ
115255; 0.8 km. S Lerdo de Tejada, UMMZ 122778; *3.6 km. NE
Minatítlán, TNHC 25150-2; 1.9 km. S Naranja, UMMZ 115253 (3); 4.5 km.
NE Novillero, UMMZ 115256; San Andrés Tuxtla, FMNH 113124-8, UIMNH
20942-3. Yucatán: Chichén-Itzá, FMNH 36570, MCZ 2463 (2).
British Honduras: Cayo: 6.2 km. S El Cayo, MCZ 37885-92. Stann Creek:
Stann Creek, FMNH 49068.
Guatemala: Alta Verapaz: 28.3 km. N Campur, KU 64578-90; Chinajá, KU
57425; Cubilquitz, UMMZ 90887, 90888 (4); Finca Chamá, UMMZ 90879
(13), 90880 (4), 90881, 90882 (28), 90883 (12), 90884 (46), 90885
(39), 90886 (20); *Finca Tinaja, BYU 16032; Panzós, UMMZ 90889 (2).
Chiquimula: Chiquimula, UMMZ 98113; 2 km. N Esquipulas, UMMZ 106844.
El petén: La Libertad, KU 57447-97, 59907-11 (skeletons), MCZ 21461,
UMMZ 75332 (13), 75333 (11), 75334 (14), 75335 (10); Piedras Negras,
FMNH 113123, UIMNH 20966; *5 km. S Piedras Negras, USNM 114951-72;
Tikal, UMMZ 117981 (2); Toocog, 15 km. SE La Libertad, KU 57426-46. El
Quiché: Finca Tesoro, UMMZ 89165 (5). Huehuetenango: Finca San Rafael,
16 km. SE Barillas, FMNH 40917-9. Izabal: Puerto Barrios, FMNH
20004-7; 8 km. S Puerto Barrios, KU 57507-37, 59991 (eggs), 59992
(tadpoles); Quirigua, CAS 69657-701; 2.5 km. NE Río Blanco, KU 57539;
San Felípe, FMNH 35065. Zacapa: 14 km. ENE Mayuelas, KU 57502-6; 8 km.
ENE Río Hondo, KU 57498-501.
Honduras: Atlantidad: La Ceiba, UMMZ 91948 (2), USNM 117593-600;
Lancetilla, MCZ 17981. Cortes: Lago Yojoa, AMNH 54917-9, 54957, 55134,
KU 64563-77. El Paraiso: Valle de Jamastran, AMNH 54807-12.
Francisco-Moranza: El Zamorano, AMNH 54873-81, KU 103223, UMMZ 123101;
Montaña de Guaimaca, AMNH 54900-4 (8); Ranch San Diego, 19 km. SW
Guaimaca, AMNH 53939. Itibucá: Vieja Itibucá, AMNH 54912-3.
Nicaragua: Chontales: 3 km. SW Santo Tomás, KU 64770-9, 68308
(skeleton). Esteli: Finca Venecia, 7 km. N, 16 km. E Condega, KU
85296; 2.4 km. N Estelí, MCZ 28933-7. MANAGUA: 12-13 km. E
Managua, KU 85297-301; *10 km. SW Tipitapa, UMMZ 119977 (10).
Matagalpa: *Finca Tepeyac, 10.5 km. N, 9 km. E Matagalpa, KU 85302-3;
Hacienda La Cumplida, KU 64780-96, 68309-11 (skeletons), UMMZ 116482
(8), 116483 (23), 116484 (3), 116485 (5), 119984 (3). Rivas: *Finca
Amayo, 13 km. S, 14 km. E Rivas, KU 85304-7; 16 km. S Rivas, MCZ
29011-7; *20.5 km. SE Rivas, KU 85308-10; 5 km. SE San Pablo, KU
43111-4.
Costa Rica: Guanacaste: Arenal, USC 6254 (2); *3 km. W Bagaces, USC
7019 (10); *3 km. NE Boca del Barranca, USC 8017 (21), *Finca San
Bosco, USC 6272 (6), 6276 (3); *Guayabo de Bagaces, USC 7022 (4), 7023
(3), 7025; 12 km. S La Cruz, USC 8091 (2); *Laguna Arenal, USC 6262;
*27 km. N Las Cañas, USC 8171 (6); *16 km. E Las Cañas, KU 102252-8;
16 km. SSE Las Cañas, KU 65090-5; *20 km. SE Las Cañas, KU 102251;
Liberia, KU 30827-39; *7.3 km. N Liberia, USC 8096 (4); *10 km. N
Liberia, USC 8085 (9); *7.5 km. SE Liberia, KU 65102-8, 68621-2
(skeletons); *14.7 km. S Liberia, USC 8238 (3); *4 km. W Liberia, KU
36847-57; 2 km. S Nicoya, USC 8230; *3-10 km. ESE Playa del Coco, USC
8012 (16), 8137 (14); *21.6 km. ESE Playa del Coco, USC 8138 (13);
*Peñas Blancas, KU 102247-50; *Río Bebedero, 5 km. S Bebedero, KU
65089; *Río Higuerón, USC 7168 (2); Santa Cruz, USC 8232 (2);
*Silencio, USC 6248; *Tenorio, KU 32313; Tilarán,
[Pg 534]
KU 36858-60; *2 km. E Tilarán, KU 86403, *5 km. NE Tilarán, KU
36840-6 USC 6269. Puntarenas: Barranca, KU 32305-12, *5 km. WNW
Barranca, UMMZ 119976 (2); *10 km. E Esparta, KU 86400-2; 1 km.
WNW Esparta, KU 65101; *4 km. WNW Esparta, KU 65088; *10 km. WNW
Esparta, KU 65063-87, 68616-20 (skeletons); *12 km. WNW Esparta,
KU 65096-100, USC 8251; 21.8 km. W San Ramón, USC 8242 (15).
Hyla robertmertensi Taylor, Proc. Biol. Soc. Washington, 50:43, April 21,
1937 [Holotype.—CNHM 100096 (formerly EHT-HMS 2270) from
Tapachula, Chiapas, México; H. M. Smith and E. H. Taylor collectors].
Smith and Taylor, Bull. U. S. Natl. Mus., 194:84, June 17, 1948; Univ.
Kansas Sci. Bull., 33:326, March 20, 1950. Mertens. Senckenbergiana,
33:170, June 15, 1952; Senckenbergischen Naturf. Gesell., 487:30, December
1, 1952. Stuart, Contr. Lab. Vert. Biol., Univ. Michigan, 68:47,
November, 1954. Duellman, Univ. Kansas Publ., Mus. Nat. Hist., 13:63,
August 16, 1960. Duellman and Hoyt, Copeia, 1961 (2): 417, December
19, 1961. Porter, Herpetologica, 18:168, October 17, 1962. Stuart,
Misc. Publ. Mus. Zool., Univ. Michigan, 122:36, April 2, 1963. Duellman
and Trueb, Univ. Kansas Publ., Mus. Nat. Hist., 17:348, July 14, 1966.
Diagnosis.—Brown lateral stripe wide, including loreal region and entire
tympanum, extending to groin, bordered above by narrow white line; dorsum
unicolor or with pair of dark lines (or rows of dashes) usually extending only
to the sacral region; shanks having dark flecks, no transverse bars; interorbital
bar lacking.
Description and Variation.—Males attain a maximum snout-vent length of
26.4 mm. in Oaxaca, whereas in a sample from Acacoyagua, Chiapas, the
largest male has a snout-vent length of 25.7 mm., and from La Trinidad,
Guatemala, 24-6 mm. Specimens from the western part of the range (eastern
Oaxaca) have slightly smaller heads and proportionately larger tympani than
the more eastern populations (Table 1).
The color pattern shows little variation, except in the nature of the dorsal
markings. In a few specimens from throughout the range, but especially in
the eastern part of the range, the dorsum lacks markings between the dorsolateral
white lines. In most specimens the dorsal pattern consists of flecks or dashes
arranged in two parallel longitudinal rows, and in some specimens the marks
are fused into parallel lines. Small brown flecks are present on the dorsal surfaces
of the shanks; in some specimens these flecks tend to form a longitudinal
stripe on the shank. An interorbital dark mark is invariably absent.
When active at night Hyla robertmertensi is pale yellow above with a white
dorsolateral line and pale brown lateral stripe; the dorsal markings are faint.
By day the dorsum is yellowish tan with brown markings. The dorsolateral
stripe is creamy white, and the lateral stripe is dark brown (Pl. 14). The
venter is white, and the iris is dull bronze. In breeding males the vocal sac
is yellow.
Remarks.—Although this species superficially resembles Hyla
microcephala microcephala, the latter is easily distinguished by the
narrow brown lateral stripe, as compared with the much wider stripe
in H. robertmertensi. No other hylids in northern Central America
and southern México can be confused with this species.
Distribution.—Hyla robertmertensi inhabits the Pacific slopes (to elevations
of 700 meters) and lowlands from eastern Oaxaca (east of the Plains of Tehuantepec)
[Pg 535]
southeastward to central El Salvador. The species also occurs in the
Cintalapa Valley (Atlantic drainage) in southwestern Chiapas (Fig. 2.) The
distribution seems to be limited on the northwest and southeast by arid environments.
The region in which Hyla robertmertensi lives is characterized by
higher rainfall and more luxuriant vegetation than occur on the Plains of
Tehuantepec or on the Pacific lowlands of eastern El Salvador and southern
Honduras. In addition to the localities listed below, Mertens (1952:30)
recorded the species from Hacienda Cuyan-Cuya, Depto. Sonsonate, El
Salvador.
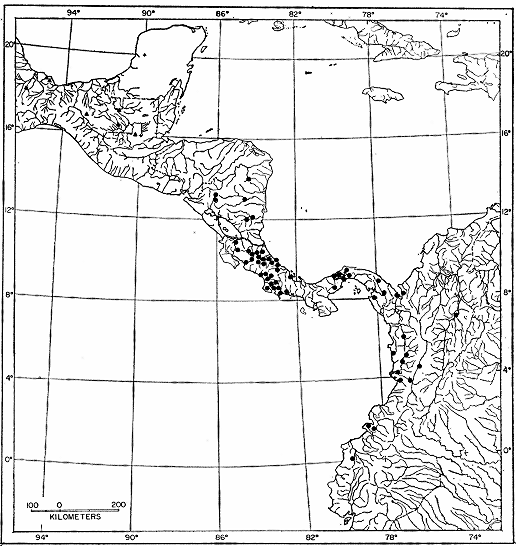
Fig. 2. Map showing locality records for Hyla robertmertensi.
Specimens examined.—490, as follows: Mexico: Chiapas: Acacoyagua,
USNM 114754-61; *2 km. W Acacoyagua, UMMZ 87843 (28), 87844 (50),
87845 (50), 87846 (45), 87847 (27), 87848 (3); 32 km. N Arriaga, KU
57619-24, 59917-8 (skeletons); Asunción, FMNH 100413, 100501-4, UIMNH
26989-90, USNM 134267; *La Esperanza, USNM 114737-48, 114750-3, 17 km.
S Las Cruces, KU 57625-49, 59997 (eggs); 8.5 km. N Puerto Madero, UMMZ
119981 (2); *11.7 km. N Puerto Madero, UMMZ 119982; Tapachula, FMNH
100096, UIMNH 26987; *11 km. S Tapachula, KU 57605-18, 59916 (skeleton);
Tonolá, FMNH 27073, 100505-10, UIMNH 26988. Oaxaca: Tapanatepec,
UMMZ 115245 (2), *1.6 km. E Tapanatepec, UMMZ 115244 (14); *4.3 km.
E Tapanatepec, UIMNH 38368-9; *7.5 km. W Tapanatepec, UMMZ 115246
(39); 12.8 km. W Tapanatepec, KU 65007-14; 7.2 km. WNW Zanatepec,
UMMZ 115243 (77); *13.6 km. WNW Zanatepec, TNHC 25213-22; 22.7 km.
WNW Zanatepec, TNHC 25203-9.
Guatemala: Jutiapa: Jutiapa, UMMZ 106848; La Trinidad, UMMZ
107733 (23). Retalhueleu: Casa Blanca, UMMZ 107732.
El Salvador: La Libertad: 16 km. NW Santa Tecla, KU 44112. San
Salvador: 21.9 km. N San Salvador, UMMZ 119983 (6).
[Pg 536]
Hyla phlebodes Stejneger, Proc. U. S. Natl. Mus., 30:817, June 4, 1906
[Holotype.—USNM 2997 from "San Carlos," Costa Rica; Burgdorf and
Schild collectors]. Taylor, Proc. Biol. Soc. Washington, 50:44, April 21,
1937; Univ. Kansas Sci. Bull., 35:888, July 1, 1952; Univ. Kansas Sci.
Bull., 39:25, November 18, 1958. Fouquette, Evolution, 14:484, December
16, 1960. Duellman and Trueb, Univ. Kansas Publ., Mus. Nat.
Hist., 17:348, July 14, 1966.
Hyla underwoodi, Dunn, Occas. Papers Boston Soc. Nat. Hist., 5:413,
October 10, 1931; Occas. Papers Boston Soc. Nat. Hist. 8:72, June 7, 1933;
Amer. Mus. Novitiates, 747.2, September 17, 1934, Gaige, Hartweg, and
Stuart, Occas. Papers Mus. Zool., Univ. Michigan, 357:5, October 26,
1937. Breder, 1946, Bull. Amer. Mus. Nat. Hist., 86:416, August 22,
1946.
Diagnosis.—Dark brown lateral stripe, if present, usually extending only to
insertion of forearm, never posteriorly to sacral region; white line above brown
stripe absent or faint; dorsal pattern weak, usually consisting of irregular dashes
or interconnected lines; interorbital dark mark present; shanks having weakly
defined transverse bars.
Description and variation.—In the majority of specimens (70%) the lateral
dark stripe extends from the nostril to the eye and thence above the tympanum
to a point above the insertion of the arm; in 17 per cent the stripe extends to
the mid-flank, whereas in 13 per cent the stripe is absent. A narrow and faint
white line is present on the canthus in some specimens, but no distinct white
stripe is present above the lateral dark line posterior to the eye. An interorbital
bar and transverse marks on the shanks are invariably present. The
dorsal markings are variable, but in most specimens (92%) consist of either an
X- or )(-shaped mark in the scapular region; in the other specimens the markings
are irregular short lines or absent. Approximately equal numbers of
specimens have a transverse bar, chevron, or broken lines in the sacral region,
whereas about eight per cent of the specimens lack markings in the sacral
region.
When active at night, individuals are pale yellowish tan with faint brown
dorsal markings. By day they are tan with more distinct brown markings
(Pl. 14). The thighs are pale yellow; the belly is white. The iris is pale creamy
tan with brown flecks. In breeding males the vocal sac is yellow.
Tadpoles.—Tadpoles of this species have been found in an extensive grassy
pond at Puerto Viejo, Costa Rica. The following description is based on KU
104099, a specimen in development stage 36 (Gosner, 1960).
Total length, 21.0 mm.; body length, 6.7 mm.; body slightly wider than
deep, snout pointed; nostrils large, directed anteriorly, situated near end of
snout; eyes small, situated dorsolaterally, directed laterally; spiracle sinistral,
located just posteroventral to eye; anal tube dextral. Tail xiphicercal; caudal
musculature moderately deep, extending far beyond posterior edge of fins; fins
deepest at about midlength; dorsal fin extending onto body, slightly deeper than
caudal musculature; ventral fin slightly shallower than musculature. Mouth
small, terminal, lacking teeth and fringing papillae, but having finely serrate
beaks. In preservative top of head olive-tan with brown flecks; dark stripe
from snout through eye to posterior edge of body; belly white, flecked with
brown anteriorly; tail creamy tan with grayish brown blotches. In life, dorsum
of body reddish tan mottled with darker brown; lateral stripe dark brown; belly
white, mottled with brown and black; caudal musculature heavily pigmented
[Pg 537]
with grayish tan; posterior tip of tail marked with dark gray; caudal fins heavily
blotched with grayish tan; iris orange-tan peripherally, red centrally (Pl. 15).
Remarks.—This species has been confused with Hyla microcephala
underwoodi by many workers. Dunn (1931, 1933, 1934)
and Breder (1946) referred Panamanian specimens of H. phlebodes
to H. underwoodi; likewise, Gaige, Hartweg, and Stuart (1937)
made the same error. Cole and Barbour (1906) and Kellog (1932)
used the name H. phlebodes for Mexican specimens of H. microcephala
underwoodi. The similarity in color pattern of H. microcephala
underwoodi and H. phlebodes easily accounts for the misapplication
of names. Although both species have nearly identical
dorsal color patterns, that of H. microcephala underwoodi usually
is bolder. Furthermore, in that species a narrow white line usually
is present above the well-defined lateral dark stripe, whereas the
lateral dark stripe is short and posterior to the eye is not bordered
above by a white line in H. phlebodes.
The type locality "San Carlos, Costa Rica" given by Stejneger
(1906:817) apparently refers to a region, the Llanuras de San Carlos,
in the northern part of Alajuela Province, Costa Rica.
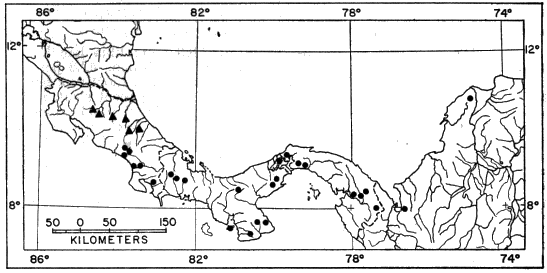
Fig. 3. Map showing locality records for Hyla phlebodes.
Distribution.—Hyla phlebodes inhabits humid tropical forests from southeastern
Nicaragua southeastward on the Caribbean slopes and lowlands to the
Canal Zone in Panamá, thence eastward in the Chucunaque Basin of eastern
Panamá and onto the Pacific lowlands of Colombia (Fig. 3). The species also
[Pg 538]
reaches the Pacific slopes in the Arenal Depression in northwestern Costa Rica
and in the Panamanian isthmus, where it occurs in humid forests on the Pacific
slope of El Valle and Cerro La Campana. Mostly the species is found at low
elevations, but it occurs at 600 meters at Turrialba and at 700 meters at Finca
San Bosco in Costa Rica.
Specimens examined.—410, as follows: Nicaragua: Zelaya: Isla Grande
del Maíz, MCZ 14848; Río Mico, El Recrero, UMMZ 79720 (6).
Costa Rica: Alajuela: 12.4 km. N Florencia, MVZ 76108-10, USC 2628;
*Las Playuelas, 11 km. S Los Chiles, USC 7216; Los Chiles, USC 7217, 7219;
3 km. NE Muelle de Arenal, USC 2644 (2); *"San Carlos," USNM 29970.
Cartago: Chitaría, KU 103690; *1.6 km. E Río Reventazón Bridge, east of
Turrialba, UMMZ 119978 (2); *Tunnel Camp, near Peralta, KU 32456,
32458-69, 41098 (skeleton); Turrialba, FMNH 101794, 103188-9, KU 25725-9,
32439-48, 41095-7 (skeletons), 64797-827, 68300-2 (skeletons), 68403 (eggs),
68404 (tadpoles), MCZ 29224-5, 29310-2, UMMZ 119979 (6), USC 31, 256
(2), 458 (2), 580, 594, 599 (7), 7074 (2), USNM 29933. Guanacaste:
Arenal, USC 6254; *Finca San Bosco, USC 62724, 6276 (3), Guayabo de
Bagaces, USC 7022 (3), 7023; *Laguna Arenal, USC 6262 (4); 3 km. NE
Tilarán, USC 524; *5 km. NE Tilarán, USC 6269; *6 km. NE Tilarán, UMMZ
122653 (6), S-2680 (skeleton), USC 523 (8). Heredia: Puerto Viejo, KU
64828-63, 68303-7 (skeletons), 68405-6 (tadpoles), 104099-100 (tadpoles);
*1.5 km. N Puerto Viejo, KU 64871; *1 km. S Puerto Viejo, KU 86432-40;
*4.2 km. W Puerto Viejo, KU 64864-5; *5.9 km. W Puerto Viejo, KU 64866-70;
*7.5 km. W Puerto Viejo, KU 86431. Limón: Batán, UMMZ 119980 (2); La
Castilla, ANSP 23707; Puerto Limón, KU 32449-55.
Panama: Bocas del Toro: 3.2 km. NW Almirante, KU 96026; Cayo de
Agua, KU 96027-31; Fish Creek, KU 96032-4. Canal Zone: Barro Colorado
Island, AMNH 69790, ANSP 23244-50; FMNH 13380, 22972-4; Juan Mina,
AMNH 55429, UU 3899; *8.6-13.8 km. N Miraflores Locks, TNHC 23439,
23477, 23484-8, 23491, 23494-9, 23501-2, 23504-8, 23510-17, 23519-30,
23532-8, 23541-54, 23561. *Rio Chagres, AMNH 55431-4; Río Cocolí, 3.5 km.
N Miraflores Locks, TNHC 23461, 23489-90, 23493, 23500, 23503, 23509,
23518, 23531, 23539-40; *Summit, ANSP 23361, KU 97788; *Three Rivers
Plantation, SU 2130. Coclé: El Valle de Antón, AMNH 55435, 69786-9,
ANSP 23506-9. Colón: Achiote, KU 77215-78; Ciricito, CAS 71499-500,
71505-6. Darién: Río Canclon at Río Chucunaque, UMMZ 126733; Río
Chucunaque, near Yavisa, AMNH 51783. Panamá: Cero La Campana, FMNH
67847-50.
Colombia: Chocó: Andagoya, FMNH 81856; Boca de Raspadura, AMNH
13570-8.
Hyla underwoodi (in part), Smith and Taylor, Bull. U. S. Natl. Mus., 194:85,
June 17, 1948.
Hyla microcephala sartori Smith, Herpetologica, 7:186, December 31, 1951
[Holotype.—UIMNH 20934 from 1 mile north of Organos, south of El
Treinte, Guerrero, México; H. M. Smith and E. H. Taylor collectors].
Duellman, Univ. Kansas Publ., Mus. Nat. Hist., 15:124, December 20,
1961. Porter, Herpetologica, 18:168, October 17, 1962. Davis and Dixon,
Herpetologica, 20:230, January 25, 1965. Duellman, Univ. Kansas Publ.
Mus. Nat. Hist., 15:652, December 30, 1965.
Diagnosis.—Dorsum tan with broad dark brown chevrons or transverse
bars; shanks marked with two or three broad transverse bars; dorsolateral
stripes absent.
Description and variation.—No noticeable geographic variation is apparent
in either structural features or coloration in this species. All specimens lack
a dorsolateral dark stripe and white line, although a dark line is present on the
[Pg 539]
canthus and dissipates in the loreal region. A broad interorbital brown bar is
present in all specimens. The color pattern on the dorsum invariably consists
of a broad, dark, chevron-shaped mark in the scapular region and a broad
dark chevron or transverse bar in the sacral region. The shanks invariably
have two or three dark brown transverse bars.
When active at night individuals are yellowish tan above with chocolate
brown markings (Pl. 14). The belly is white, and the thighs are pale yellowish
tan. The iris is dark bronze-color. In breeding males the vocal sac is yellow.
By day some individuals were observed to change to creamy gray with distinct
darker markings.
Remarks.—Although tadpoles of this species have not been found,
observations on the breeding sites indicate that the tadpoles probably
develop in ponds. Except for calling males observed around a
pool in a stream-bed 11.8 kilometers west-northwest of Tierra Colorada,
Guerrero, all breeding congregations have been found at
temporary ponds.
Smith (1951:186) named Hyla sartori as a subspecies of Hyla
microcephala. This subspecific relationship seemed reasonable until
analysis of the mating calls showed that the call of H. sartori is more
nearly like that of H. phlebodes than that of H. microcephala. The
broad hiatus separating the ranges of H. microcephala and H. sartori
is additional evidence for considering H. sartori as a distinct species.
Fig. 4. Map showing locality records for Hyla sartori.
Distribution.—Hyla sartori occurs in mesophytic forests to elevations of
about 300 meters on the Pacific slopes of southern México from southwestern
Jalisco to south-central Oaxaca (Fig. 4). The lack of specimens from Colima
and Michoacán probably reflects inadequate collecting instead of the absence
of the species there. On the basis of available habitat the species would be
expected to occur in Nayarit, but extensive collecting there has failed to
reveal its presence. The semi-arid Plains of Tehuantepec apparently limit the
distribution to the east.
[Pg 540]
Specimens examined.—190, as follows: México: Guerrero: 5 km. E Acapulco,
AMNH 54611-2; 23.2 km. N Acapulco, UIMNH 26404-7; Colonia Buenas
Aires, 23 km. E Tecpán de Galeana, UMMZ 119223 (7); *El Limoncito,
FMNH 75785, 100390-402, 104631, 104633, UMMZ 117250, USNM 134266;
El Treinte, FMNH 100403, UIMNH 20935-7; Laguna Coyuca, AMNH
59686; La Venta, MCZ 29635; *Morjonares, UIMNH 26392-402; 1.6 km.
N Organos, FMNH 100404-5, UIMNH 20933-4; 19.2 km. S Petaquillas,
UIMNH 26408; 6.1 km. E. Tecpán de Galeana, TNHC 23396-408; *11.2 km.
N Tierra Colorada, UIMNH 26403; 11.8 km. WNW Tierra Colorada, UMMZ
119225 (51), S-2677-9 (skeletons); Zacualpán, UMMZ 119224 (6). Jalisco:
6.4 km. NE La Resolana, KU 67853-69; 24 km NE La Resolana, KU 67870-3.
Oaxaca: 3 km. N Pochutla, KU 57539; 13.4 km. N Pochutla, UMMZ
123495 (40).
The frogs of the Hyla microcephala group have a minimal amount
of cranial ossification as compared to more generalized hylid skulls,
such as Smilisca (Duellman and Trueb, 1966). In the Hyla microcephala
group the sphenethmoid is small and short, and a large
frontoparietal fontanelle is present. The quadratojugal exists only
as a small spur and is not in contact with the maxillary. The
proötics are poorly developed. The anterior and posterior arms
of the squamosal are short; the anterior arm extends no more than
one-fourth of the distance to the maxillary, and the posterior arm
does not have a bony connection with the proötic. The nasal lacks
a maxillary process, and the medial ramus of the pterygoid lacks a
bony connection to the proötic.
Teeth are absent on the parasphenoid and palatines, but present
on the maxillaries, premaxillaries, and prevomers. The teeth are
simple, pointed, and slightly curved. Although the number of teeth
varies (Table 3), no consistent differences between the species are
apparent.
| Species | N | Maxillary | Premaxillary | Prevomer |
| H. microcephala | 32 | 31-47(37.8) | 4-13(8.9) | 2-4(3.2) |
| H. phlebodes | 10 | 38-45(40.1) | 8-13(10.3) | 2-5(3.9) |
| H. robertmertensi | 6 | 23-43(32.8) | 7-12(10.5) | 2-3(2.7) |
| H. sartori | 6 | 27-43(38.2) | 9-10(9.3) | 3-4(3.7) |
PLATE 13
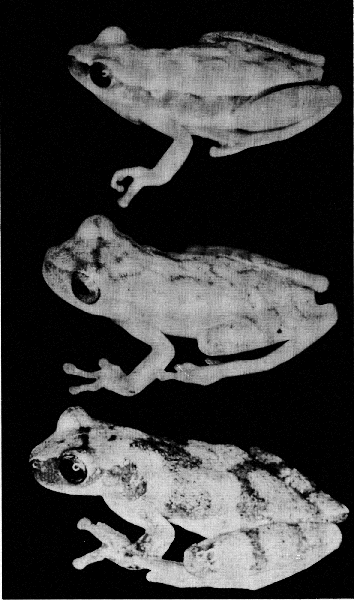
Upper figure, Hyla microcephala microcephala (KU 64593);
middle figure, H. microcephala underwoodi (KU 64565);
lower figure, H. microcephala underwoodi (UMMZ 115247).
All approximately ×3.
PLATE 14
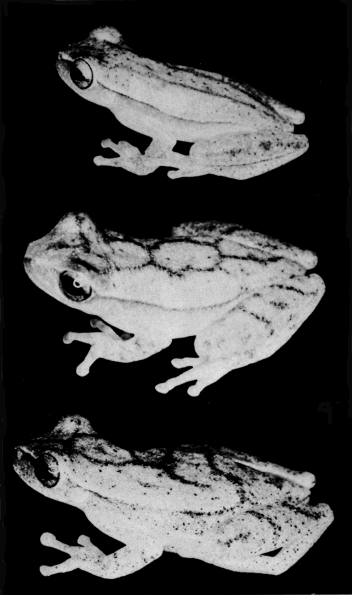
Upper figure, Hyla robertmertensi (UMMZ 115243);
middle figure, H. phlebodes (KU 64798);
lower figure, H. sartori (UMMZ 119225).
All approximately ×3.
PLATE 15
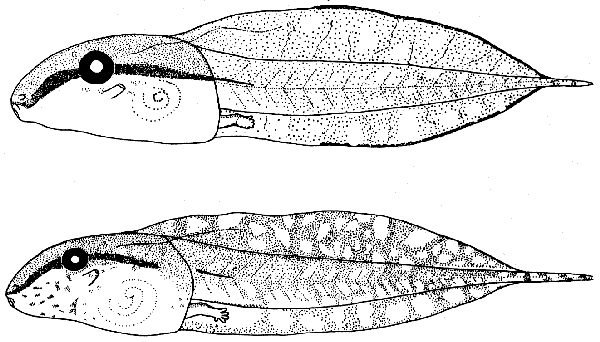
Tadpoles of Hyla microcephala group:
upper figure, H. m. microcephala (KU 104097);
lower figure, H. phlebodes (KU 104099).
Both ×4.
PLATE 16
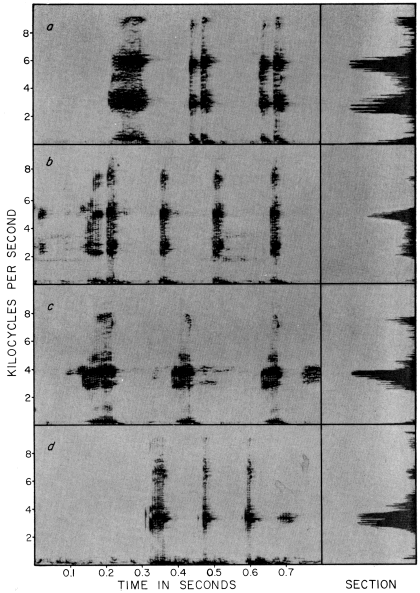
Audiospectrograms and sections of mating calls of Hyla microcephala group:
(a) H. m. microcephala (KU Tape No. 19);
(b) H. robertmertensi (KU Tape No. 41);
(c) H. phlebodes (KU Tape No. 6);
(d) H. sartori (KU Tape No. 190).
| Character | H. microcephala | H. robertmertensi | H. phlebodes | H. sartori |
| Frontoparietal | Minimally ossified with large fontanelle extending from sphenethmoid to occipital ridge. | Ossification extensive anteriorly with narrow medial separation; fontanelle largest in parietal region. | Ossification extensive anteriorly with narrow medial separation; fontanelle largest in parietal region. | Ossification moderately extensive anteriorly; medial separation of about uniform width throughout length of fontanelle. |
| Nasals | Moderately long and slender; arcuate in dorsal view. | Moderate in size; slightly wider anteriorly than posteriorly in dorsal view. | Moderate in size; slightly wider anteriorly than posteriorly in dorsal view. | Long and broad; arcuate in dorsal view. |
| Sphenethmoid | Extremely short in dorsal view. | Moderately short in dorsal view. | Moderately short in dorsal view. | Moderately short in dorsal view; ossified anteriorly between nasals. |
| Columella | Distal and greatly expanded. | Distal and slightly expanded or not. | Distal and not expanded. | Distal and not expanded. |
[Pg 542]
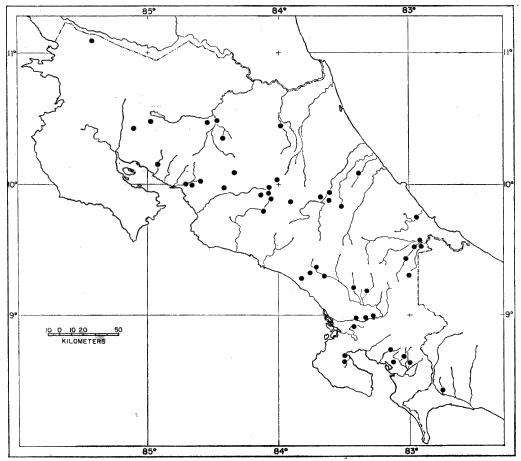
Fig. 5.Dorsal views of the skulls of (a) Hyla m. microcephala
(KU 68293) and (b) H. sartori(UMMZ S-2677). Both × 12.
[Pg 543]
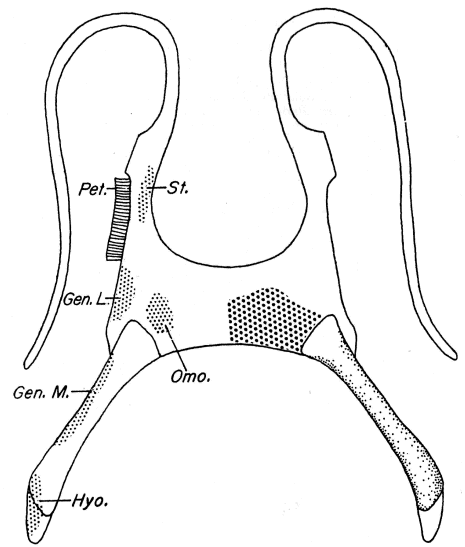
Fig. 6. Dorsal views of skulls of (a) Hyla phlebodes (KU 68303)
and (b) H. robertmertensi (KU 59917). Both × 12.
Despite the great reduction in the ossification of the cranial
elements, certain apparently consistent differences exist between
[Pg 544]
the species seem to be consistent. The most notable differences
are: 1) amount of ossification of the frontoparietals and consequent
shape and size of the frontoparietal fontanelle, 2) shape of the
nasals, 3) shape and extent of the sphenethmoid, and 4) shape of
the columella (Table 4, Figs. 5-6). On the basis of these characters,
Hyla microcephala can be set apart from the other species and
characterized as having a poorly ossified frontoparietal and correspondingly
large frontoparietal fontanelle; long, slender, arcuate
nasals; extremely short sphenethmoid; and expanded distal end of
the columella. The other species in the group (phlebodes, robertmertensi,
and sartori) have more ossification of the frontoparietals,
broader nasals, only a moderately short sphenethmoid, and an unexpanded
distal end of the columella. Among these three species,
the skulls of phlebodes and robertmertensi are most nearly alike,
whereas the skull of sartori differs by having a differently shaped
frontoparietal fontanelle, broader nasals, and an ossified anterior
extension of the sphenethmoid between the nasals (compare Fig. 5b with Fig. 6 a-b).
Although all skulls examined belong to breeding adults, the
extent of the ossification of the frontoparietals and the resulting
shape of the frontoparietal fontanelle might be correlated with the
age of the frog. Nevertheless, in the 24 skulls of Hyla microcephala
examined, the frontoparietals are less extensively ossified than in
the skulls of the other species. The trivial differences among the
other three species certainly are suggestive of close relationship,
but on the basis of present knowledge of the evolutionary trends
in hylid cranial osteology, the differences offer little evidence for
determining phylogenetic lineage.
Calls of all five taxa were compared in several characteristics, of
which three are deemed most significant systematically. These
are 1) the pattern and duration of the notes of a call-group, 2) the
fundamental frequency, and 3) the dominant frequency. Air temperatures
were noted at the time the calls were recorded, but no
valid correlation could be determined between this factor and any
of the parameters of the calls; consequently recordings made at all
temperatures (21-29° C.) were grouped together.
Pattern and duration of notes.—In all five taxa the basic pattern
consists of a call-group made up of one primary note followed by
a series of shorter secondary notes. In some species the secondary
[Pg 545]
notes differ from the primary in other characteristics. Both subspecies
of Hyla microcephala have a long, unpaired primary note
followed by 0 to 18 (usually about 4) somewhat shorter paired
secondary notes. In calls of Hyla m. microcephala the mean duration
of the primary is 0.131 (0.10-0.16) second and that of the
secondaries is 0.101 (0.05-0.14) second, whereas in H. m. underwoodi
the mean duration of the primary is 0.018 (0.05-0.15) second
and that of the secondaries is 0.086 (0.06-0.11) second.
Hyla robertmertensi has a reverse of this pattern in that the
primary note is paired and the secondaries are unpaired. In the
sample studied a call-group contains 0-28 secondary notes (generally
about 3). The mean duration of the primary is 0.091 (0.07-0.11)
second and that of the secondaries is 0.040 (0.025-0.06) second.
Hyla phlebodes and sartori have call-groups composed of a rather
short, unpaired primary and several short, unpaired secondaries
(0-28 in phlebodes, 0-23 in sartori). The mean duration of the
primary of phlebodes is 0.105 (0.07-0.16) second and that of the
secondaries is 0.067 (0.035-0.12) second. The mean duration of the
primary of sartori is 0.080 (0.07-0.09) second and that of the
secondaries is 0.053 (0.035-0.07) second.
The two subspecies of H. microcephala are identical in call pattern
and agree closely in duration of notes, although those of the nominate
subspecies tend to be slightly longer. Hyla robertmertensi is
distinctive in call pattern in that it is the only species having a paired
primary; the duration of the primary is completely overlapped by
that in the other species, but the secondaries tend to be the shortest
in the group. The call patterns of H. phlebodes and H. sartori are
identical and the range of duration of notes of phlebodes completely
overlaps that of sartori, although both the primary and secondary
notes of the latter tend to be somewhat shorter (Table 5, Pl. 16).
Fundamental frequency.—This parameter was analyzed for the
primary notes. It was measured for the secondaries as well and
was found to differ in magnitude in the same way as the primary
note. In a few examples of both subspecies of H. microcephala a
high primary note, in which the fundamental frequency is exceptionally
high, is sometimes emitted (Fouquette, 1960b). None of
these notes was used in this analysis; only the fundamental frequencies
of normal primary notes are compared (Table 5, Fig. 7).
Mean; Unless Otherwise Noted Data Are for Primary Notes.).
| Species | N | Dominant frequency (cps) | Fundamental frequency (cps) | Duration of notes (seconds) | Repetition rate of secondaries (notes/minute) | |
| Primary | Secondary | |||||
| H. m. microcephala | 44 | 5637 | 205 | 0.13 | 0.10 | 268 |
| (5150-5962) | (184-244) | (0.11-0.16) | (0.05-0.14) | (192-353) | ||
| H. m. underwoodi | 47 | 5772 | 220 | 0.11 | 0.09 | 283 |
| (5177-6200) | (192-275) | (0.05-0.15) | (0.06-0.11) | (197-384) | ||
| H. robertmertensi | 25 | 5388 | 162 | 0.09 | 0.04 | 418 |
| (5150-5785) | (140-178) | (0.07-0.11) | (0.03-0.06) | (368-570) | ||
| H. phlebodes | 34 | 3578 | 148 | 0.11 | 0.07 | 284 |
| (3220-4067) | (125-158) | (0.07-0.16) | (0.04-0.12) | (210-350) | ||
| H. sartori | 10 | 3217 | 126 | 0.08 | 0.05 | 434 |
| (2950-3600) | (116-135) | (0.07-0.09) | (0.04-0.07) | (396-477) | ||
The two subspecies of H. microcephala agree closely in fundamental
frequency. There is considerable overlap, but the difference
between the means is significant at the 0.001 level of probability
(t = 4.2406). The call of H. robertmertensi does not overlap that
[Pg 547]
of H. sartori or either subspecies of H. microcephala in this parameter;
but it does overlap that of H. phlebodes, although again the
difference between the means is significant at the 0.001 level
(t = 9.360). Hyla phlebodes and sartori have the lowest fundamental
frequencies, and there is some overlap, but here too the
difference between the means is significant at the 0.001 level
(t = 4.923).
Dominant frequency.—A dominant band of of frequencies cuts
across the harmonics of the fundamental, obscuring the harmonic
pattern and generally shifting upward in frequency. The midpoint
of this band is measured at the terminal border as the dominant
frequency. As with the fundamental frequency, only the normal
primary notes were utilized in the comparisons (Table 5, Fig 8).
Fig. 7. Variation in the fundamental frequency of the normal primary notes
in the Hyla microcephala group. The horizontal lines = range of variation,
vertical lines = mean, solid bars = twice the standard error of the mean, and
open bars = one standard deviation. The number of specimens in each
sample is indicated in parentheses after the name of the taxon.
The two subspecies of H. microcephala agree more closely in this
parameter than in fundamental frequency. The overlap is great,
but the difference between the means is significant at the 0.001 level
(t = 3.658). The calls of both subspecies completely overlap that
of robertmertensi in this parameter, but the difference between the
means is significant at the 0.001 level. The calls of H. phlebodes
and H. sartori overlap considerably in this characteristic, although
the difference between the means is significant at the 0.001 level
(t = 7.504) (Fig. 9). The call of neither species overlaps those
of H. microcephala and robertmertensi.
[Pg 548]
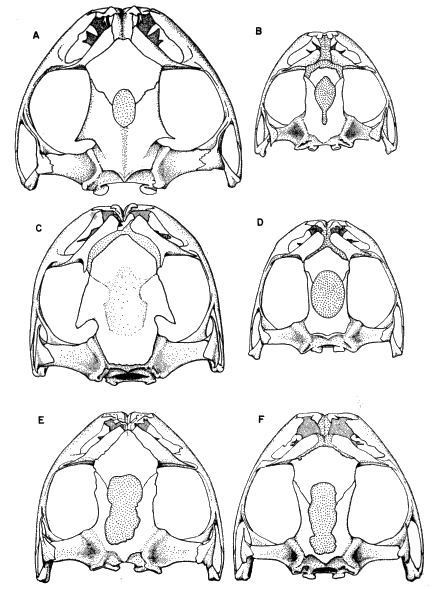
Fig. 8. Variation in the mid-point of the dominant frequency band of the
normal primary notes in the Hyla microcephala group. The horizontal lines
= range of variation, vertical lines = mean, solid bars = twice the standard
error of the mean, and open bars = one standard deviation. The number of
specimens in each sample is indicated in parentheses after the name of the
taxon.
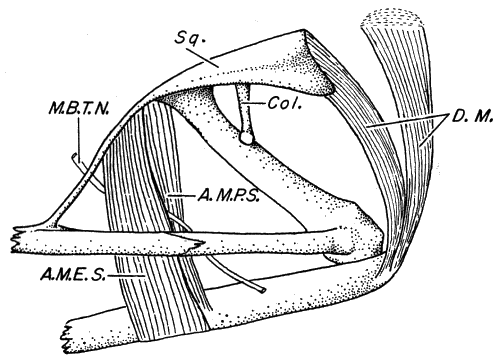
Fig. 9. Scatter diagram relating the dominant and fundamental frequencies
of the normal primary notes in the Hyla microcephala group. Each symbol
represents a different individual.
Repetition rate.—The repetition rate of the secondary notes, in
calls consisting of more than one secondary, was measured for each
form. A considerable amount of variation in this parameter was
found in all of the taxa (Table 5). This variation probably is due
in part to the effect of temperature differences. Repetition rate is
[Pg 549]
the only parameter analyzed for which there is a correlation with
the air-temperature, but even here the correlation is weak, probably
due to the microenvironmental effects of humidity, air-movement,
and other factors in addition to the ambient air temperature that
influences the body temperature of the frogs. These rates are
nearly alike in both subspecies of H. microcephala and in phlebodes.
The repetition rates in H. robertmertensi and H. sartori are considerably
faster than in the other three taxa. Hyla sartori has the
fastest repetition rate of the group.
In all characteristics of the mating calls the two subspecies of
H. microcephala agree closely, as might be expected, although the
differences are statistically significant. Hyla robertmertensi is distinctive
in call pattern and seems to be closer to microcephala in
dominant frequency but closer to H. phlebodes in fundamental frequency.
Thus, it is somewhat intermediate between microcephala
and phlebodes. The identical pattern and similarity in fundamental
and dominant frequencies of the calls of H. phlebodes and H. sartori
possibly indicate close relationship.
Geographic variation in call.—Hyla m. microcephala has higher
fundamental and dominant frequencies in Costa Rica than in Panamá.
In Costa Rican H. m. underwoodi the fundamental and dominant
frequencies are lower than in other parts of the range. Frogs of
this subspecies recorded in Nicaragua and Honduras have slightly
lower dominant frequencies and higher fundamental frequencies
than those recorded in Guatemala or Oaxaca. The duration of both
primary and secondary notes decreases to the south; samples from
Nicaragua and Costa Rica have the shortest notes. Comparison
of duration of notes in the two subspecies shows that the Panamanian
H. m. microcephala have slightly longer notes than do any
H. m. underwoodi; the more northern populations of H. m. underwoodi
from México most closely approach H. m. microcephala in
this characteristic.
The calls of H. robertmertensi in Oaxaca have higher dominant
and fundamental frequencies and longer secondary notes than do
those in Chiapas.
The calls of H. phlebodes recorded at Puerto Viejo, Costa Rica,
have slightly lower dominant frequencies than do those recorded
at Turrialba, Costa Rica, and in Panamá, whereas those recorded at
Turrialba have lower fundamental frequencies than in other samples.
The duration of notes is slightly shorter in both Costa Rican samples
than in those recorded in Panamá.
[Pg 550]
The frogs of the Hyla microcephala group breed in shallow grassy
ponds. In some places they breed in permanent ponds, but usually
congregate around temporary pools, such as depressions in forests,
flooded fields, and roadside ditches. At the height of their breeding
season, usually in the early part of the rainy season, the congregations
are made up of large numbers of individuals. In April, 1961,
and in June, 1966, the senior author noted nearly continuous
choruses of H. m. microcephala in roadside ditches along the 75
kilometers of road between Villa Neily and Palmar Sur, Puntarenas
Province, Cost Rica; on June 20, 1966, at Puerto Viejo, Heredia
Province, Costa Rica, he estimated approximately 900 Hyla phlebodes
in one pond, and two nights later noticed that the number
of individuals had increased substantially. Other observations by
the first author on size of breeding congregations include nearly
continuous choruses of H. m. underwoodi between Villahermosa and
Teapa, Tabasco, in July of 1958, an estimated 400 Hyla robertmertensi
in a road side ditch 7.2 kilometers west-northwest of Zanatepec,
Oaxaca, on July 13, 1956, and approximately 150 Hyla sartori around
a rocky pool in a riverbed, 11.8 kilometers west-northwest of Tierra
Colorada, Guerrero, on June 28, 1958.
The length of the breeding season seemingly is more dependent
on climatic conditions in various parts of Middle America than on
behavioral differences in the various species. Thus, Fouquette
(1960b) found in the Canal Zone that H. m. microcephala formed
breeding choruses from May through January, the entire rainy
season in that area. In the wetter coastal region of Puntarenas
Province, Costa Rica, the species breeds as early as mid-March,
whereas in the drier region encompassing Guanacaste Province,
Costa Rica, and southwestern Nicaragua breeding activity is initiated
by the first heavy rains of the season, usually in June.
Hyla phlebodes inhabits regions having rainfall throughout the
year. Although large breeding congregations are most common in
the early parts of the rainy season, males probably call throughout
the year. At Puerto Viejo in Costa Rica the senior author has heard
Hyla phlebodes in February, April, June, July, and August. Charles
W. Myers noted calling males of this species in the area around
Almirante, Bocas del Toro Province, Panamá, in September, October,
and February. An exception to the correlation between rainfall and
breeding activity was noted by the junior author in Hyla phlebodes
in the Canal Zone, where he noticed a decrease in activity of that
species in October and November, when the rains are heaviest and
[Pg 551]
most frequent. Furthermore, independent observations made by
both of us indicate that H. phlebodes does not reach peaks of
activity during or immediately after heavy rains, but instead builds
up to peaks of activity two or three days after a heavy rain. This
is in contrast to the other species, all of which characteristically inhabit
drier environments than does H. phlebodes. Peaks of breeding
activity in the other species occur immediately after, or even
during, heavy rains.
The calling location of the males generally is on vegetation above,
or at the edge of, the water. Hyla microcephala and H. phlebodes
call almost exclusively from grasses and sedges; phlebodes usually
calls from taller and more dense grasses than does microcephala.
Except for some minor differences in calling location observed by
the junior author (Fouquette, 1960b) in the Canal Zone, the differences
in density and height of grasses utilized for calling-locations
probably is dependent primarily on the nature of the available
vegetation. Although bushes and broad-leafed herbs are usually
present at the breeding sites, males of these species seldom utilize
them for calling locations. Both H. robertmertensi and H. sartori
have been observed calling from grasses, herbs, bushes, and low
trees. Calling males of robertmertensi have been found two meters
above the ground in small trees.
Daytime retreats in the breeding season sometimes are no more
than shaded clumps of vegetation adjacent to a pond or in clumps
of grass in a pond. Individuals of H. m. underwoodi were found by
day under the outer sheaths of banana plants next to a water-filled
ditch. Dry season refuges are unknown.
Amplexus is axillary in all four species. Egg deposition has been
observed in H. m. microcephala, m. underwoodi, and phlebodes.
In all three the eggs are deposited in small masses that float near
the surface of the water and usually are at least partly attached to
emergent vegetation. Each clutch does not represent the entire egg
complement of the female.
Tadpoles are definitely known of only H. m. microcephala and
phlebodes; these have been described in the preceding accounts of
the species. The tadpoles of these two species can be distinguished
readily (Pl. 15). The tadpole of H. microcephala has a uniformly
white venter and nearly transparent tail, whereas in H. phlebodes
the venter is flecked anteriorly and the tail is mottled. In life, H.
microcephala is easily recognized by the orange posterior half of
the tail, whereas the tail in H. phlebodes is mottled tan and grayish
brown.
[Pg 552]
The evidence already presented on osteology, external structure,
coloration, mating call, and life history emphatically show that the
four species under consideration are a closely related assemblage.
Now the question arises: To what other groups in the genus is the
Hyla microcephala group related? Furthermore, it is pertinent
to this discussion to attempt a reconstruction of the phylogeny of
the group as a whole and of the individual species in the Hyla
microcephala group. With regard to the relationships of the group
we must take into account certain species in South America. Our
endeavors there are hampered by the absence of data on the mating
calls and life histories of most of the relevant species.
As mentioned in the account of Hyla m. microcephala, the species
microcephala possibly is subspecifically related to Hyla misera, a
frog widespread in the Amazon Basin. Hyla misera resembles
microcephala in coloration, external structure, and cranial characters.
The frontoparietals are equally poorly ossified, and the frontoparietal
fontanelle is extensive. Our principal reason for not considering
the two taxa conspecific at this time is our lack of knowledge
concerning the color of living H. misera, the structure of the tadpoles,
and the characteristics of the mating call. Even with the
absence of such data that we think essential to establish the nomenclature
status of the taxa, we are confident that the two are sufficiently
closely related that any discussion of the phylogenetic relationships
of one species certainly must involve consideration of the
other.
Hyla misera possibly is allied to other small yellowish tan South
American Hyla that lack dark pigmentation on the thighs. Probable
relatives are Hyla elongata, minuta (with goughi, pallens, suturata,
velata, and possibly others as synonyms), nana, and werneri. The
consideration of the interspecific relationships of these taxa is beyond
the scope of this paper, but we can say that each of these
species has a pale yellowish tan dorsum, relatively broad dorsolateral
brown stripe, and narrow longitudinal brown lines or irregular marks
on the dorsum. Furthermore, examination of the skulls of elongata,
nana, and werneri reveals that they are like misera and microcephala
in the nature of the frontoparietal fontanelle and in having a greatly
reduced quadratojugal. Thus, on the basis of cranial and external
characters the Hyla microcephala group can be associated with Hyla
misera and its apparent allies in South America. This association
can be only tentative until the mating calls, tadpoles, and chromosome
numbers of the South American species are known.
[Pg 553]
Among the Middle American hylids, only the Hyla microcephala
group and H. ebraccata have a haploid number of 15 chromosomes
(Duellman and Cole, 1965). All other New World Hyla, for which
the number is known, have a haploid number of 12; the only other
Hyla having 15 is a Papuan Hyla angiana (Duellman, 1967).
Hyla ebraccata occurs in the humid tropical lowlands of Middle
America and the Pacific lowlands of northwestern South America.
It is the northernmost, and only Central American, representative
of the Hyla leucophyllata group, which is diverse (about 10 species
currently recognized) and widespread in tropical South America
east of the Andes. This group is characterized by having broad,
flat skulls with larger nasals and more ossification of the frontoparietals
than in the Hyla microcephala group. The quadratojugal
is present as a small anteriorly projecting spur that does not connect
with the maxillary. Externally, the Hyla leucophyllata group is
characterized by having a well-developed axillary membrane, uniformly
yellow thighs, and a dorsal color pattern in many species
consisting of a dark lateral band, a pale dorsolateral band or dorsal
ground color, and a large middorsal dark mark. In some species,
the dorsal pattern consists of small dark markings or is nearly uniformly
pale. At least in the Central American Hyla ebraccata, the
mating call consists of a single primary note followed by a series of
shorter secondary notes, the tadpoles have xiphicercal tails and lack
teeth, and the haploid number of chromosomes is 15. On the
strength of these observations it seems imperative to consider the
Hyla leucophyllata group as a close ally to the Hyla microcephala
group. Successful artificial hybridization supports the close relationship
of H. m. microcephala and phlebodes; partial success of
artificial hybridization of these two with ebraccata (Fouquette,
1960b) provides further evidence for close relationship between the
Hyla leucophyllata and Hyla microcephala groups.
In México and northern Central America two small species, Hyla
picta and Hyla smithi, comprise the Hyla picta group. These frogs
resemble members of the Hyla microcephala group by having a
yellowish tan dorsum with a dorsolateral white stripe and uniformly
yellow thighs. Furthermore the mating call is not unlike those of
the species in the Hyla microcephala group. Despite these similarities,
the Hyla picta group differs from the Hyla microcephala
group by having a well-developed quadratojugal that connects to
the maxillary, tadpoles with teeth present and caudal fins completely
enclosing the caudal musculature, and a haploid number
of 12 chromosomes. In all of these characteristics the frogs of the
[Pg 554]
Hyla picta group more closely resemble other Middle American
Hyla than they do the Hyla microcephala group. Therefore, it
can best be presumed that the superficial resemblances of coloration
and the mating call are the result of convergence.
Since the Hyla microcephala and leucophyllata groups apparently
are related and since the greatest diversity of these frogs is in
South America (if Hyla misera and its relatives are placed with the
Hyla microcephala group), it seems appropriate to place the
centers of origins of these groups in South America. Therefore,
the Hyla microcephala group and Hyla ebraccata of the Hyla leucophyllata
group either have immigrated into Central America, or
they are representatives of those groups that were isolated in
Central America during most of the Cenozoic when South America
was separated from Central America.
The interspecific relationships of the species in the Hyla microcephala
group are not clear. On the basis of coloration, H. m. microcephala
and H. robertmertensi are close, and H. m. underwoodi and
H. phlebodes are nearly identical. The mating calls of H. phlebodes
and sartori closely resemble one another, whereas the call of robertmertensi
is intermediate between these and microcephala.
In most respects Hyla microcephala is distinct from the other
species, and with the exception of the amount of ossification of the
frontoparietals, the other species can be easily derived from a
microcephala-like ancestor. Possibly the slightly increased ossification
of the frontoparietals in robertmertensi, phlebodes, and sartori
is secondary, or possibly after differentiation of the species the
amount of ossification was further reduced in microcephala. If so,
the species fall into a reasonable phylogenetic scheme that has
microcephala as the extant species most like the ancestral stock.
We visualize the evolutionary history of the group to have followed
a course that began with the invasion of Central America by
a microcephala ancestral stock that differentiated into two populations
in lower Central America—a microcephala-like frog on the
Pacific lowlands and a phlebodes-like frog on the Caribbean lowlands.
Differentiation could have been brought about by isolation
by montaine or marine barriers. The population on the Pacific
lowlands either was preadapted for subhumid conditions or became
so adapted and dispersed northward onto the Pacific lowlands of
northern Central America. Simultaneously the frogs on the Caribbean
lowlands, which were adapted to humid environments, dispersed
northward in the humid forested regions to southern México
and crossed the Isthmus of Tehuantepec onto the Pacific slopes of
[Pg 555]
Oaxaca and Guerrero northward to Jalisco. Subsequent development
of arid conditions, possibly in the Pliocene, Pleistocene, or even as
late as the Thermal Maximum in post-Wisconsin time, resulted in
a restriction of the ranges in northern Central America, thereby
isolating part of the phlebodes-stock on the Pacific slopes of México,
where it adapted to drier conditions and evolved into sartori. The
rest of the phlebodes-stock was restricted to the humid forests on
the Caribbean lowlands of lower Central America. The increased
aridity on the Pacific lowlands eliminated the microcephala-stock
from southern Honduras and northwestern Nicaragua and in so
doing left an isolated population on the lowlands of Chiapas and
Guatemala, which differentiated into robertmertensi. The original
stock on the Pacific lowlands of Panamá and southeastern Costa
Rica became microcephala.
If the microcephala-stock was, as we believe, better adapted for
existence under subhumid conditions than was the phlebodes-stock,
the development of subhumid conditions in much of the lowland
region of northern Central America and southern México would
have permitted the expansion of the range of microcephala into the
area now inhabited by H. m. underwoodi, while phlebodes was
being eliminated from this area by climatic conditions that were
unsuited to its survival there. Perhaps the similarity in coloration
of H. m. underwoodi and phlebodes is the result of convergence or
possibly hybridization occurred at the time the former was expanding
its range and the latter's range was being restricted. If hybridization
did occur, the differences in mating call subsequently
were enhanced, thereby providing a valid isolating mechanism in
sympatric populations.
Hyla microcephala and phlebodes range into northern South
America. Probably both species entered South America in relatively
recent times after they had differentiated from one another in
Central America.
[Pg 556]
1898. Fourth report on additions to the batrachian collection in the Natural-History Museum. Proc. Zool. Soc. London, 1898, pp. 373-482, pls. 38-39. October 1.
1899. Descriptions of new batrachians in the collection of the British Museum (Natural History). Ann. Mag. Nat. Hist, ser. 7, 3:273-277, pls. 11-12.
1946. Amphibians and reptiles of the Rio Chucunaque Drainage, Darien, Panama, with notes on their life histories and habits. Bull. Amer. Mus. Nat. Hist, 86:375-436, pls. 42-60, August 26.
1906. Vertebrata from Yucatan: Reptilia; Amphibia; Pisces. Bull. Mus. Comp. Zool., 50:146-159. November.
1886. Thirteenth contribution to the herpetology of tropical America. Proc. Amer. Philos. Soc, 23:271-287. February 11.
1894. Third addition to a knowledge of the Batrachia and Reptilia of
Costa Rica. Proc. Acad. Nat. Sci. Philadelphia, 1894, pp. 194-206.
1956. The frogs of the hylid genus Phrynohyas Fitzinger, 1843. Misc. Publ. Mus. Zool., Univ. Michigan, 96:1-47, pls. 1-6. February 21.
1967. Additional studies of chromosomes of anuran amphibians. Syst. Zool., 16:38-43, March 17.
1965. Studies of chromosomes of some anuran amphibians (Hylidae and Centrolenidae). Syst. Zool., 14:139-143. July 9.
1966. Neotropical hylid frogs, genus Smilisca. Univ. Kansas Publ., Mus. Nat. Hist., 17:281-375, pls. 1-12. July 14.
1931. The amphibians of Barro Colorado Island. Occas. Papers Boston Soc. Nat. Hist., 5:403-421. October 10.
1933. Amphibians and reptiles from El Valle de Anton, Panamá. Ibid., 8:65-79. June 7.
1934. Two new frogs from Darien. Amer. Mus. Novit., 747:1-2. September 17.
1960a. Call structure in frogs of the family Leptodactylidae. Texas Jour. Sci., 12:201-215. October.
1960b. Isolating mechanisms in three sympatric tree frogs in the Canal Zone. Evolution, 14:484-497. December 16.
1937. Notes on a collection of amphibians and reptiles from eastern Nicaragua. Occas. Papers Mus. Zool., Univ. Michigan, 357:1-18. October 26.
1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16:183-190. September 23.
[Pg 557]
1932. Mexican tailless amphibians in the United States National Museum. Bull. U.S. Natl. Mus., 160:1-224. March 31.
1961. Salientia of Venezuela. Bull. Mus. Comp. Zool., 126:1-207. November.
1951. The identity of Hyla underwoodi Auctorum of Mexico. Herpetologica, 7:184-190. December 31.
1906. A new tree toad from Costa Rica. Proc. U. S. Natl. Mus., 30:817-818. June 4.
1935. A contribution to a knowledge of the herpetology of a portion of the savanna region of central Petén, Guatemala. Misc. Publ. Mus. Zool., Univ. Michigan, 29:1-56, pls. 1-4. October 4.
1952. The frogs and toads of Costa Rica. Univ. Kansas Sci. Bull., 35-577-942. July 1.
Transmitted July 11, 1967.
Featured Books

The Turn of the Screw
Henry James
have to wait. We waited in fact till two nightslater; but that same evening, before we scattered, h...

Two New Moles (Genus Scalopus) from Mexico and Texas
Rollin H. Baker
ecies which may be named and described asfollows:Scalopus montanus new speciesType.—Male, adult, s...

Fish Populations, Following a Drought, in the Neosho and Marais des Cygnes Rivers of Kansas
James E. Deacon
s.Published August 11, 1961 University of KansasLawrence, Kansas PRINTED INTHE STATE PRINTING ...

Birds, Illustrated by Color Photography, Vol. 2, No. 5
Various
ct in my memory as if it hadoccurred this very day, I have thoughtthousands of times since, and will...

Jimgrim and Allah's Peace
Talbot Mundy
lid black; /* a thin black line border.. */ padding: 6px; /* ..spaced a bit out from the gr...

The call of the wild
Jack London
ts four sides. The house was approached by gravelleddriveways which wound about through wide-spreadi...

A Columbus of Space
Garrett Putman Serviss
lid black; /* a thin black line border.. */ padding: 6px; /* ..spaced a bit out from the gr...

The Time Traders
Andre Norton
ively; his unlined boy's face was not one tobe remembered—unless one was observant enough to note ...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Middle American Frogs of the Hyla microcephala Group" :