The Project Gutenberg eBook of Myology and Serology of the Avian Family Fringillidae: A Taxonomic Study
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Myology and Serology of the Avian Family Fringillidae: A Taxonomic Study
Author: William B. Stallcup
Release date: October 19, 2010 [eBook #33914]
Language: English
Credits: Produced by Chris Curnow, Tom Cosmas, Joseph Cooper and
the Online Distributed Proofreading Team at
https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK MYOLOGY AND SEROLOGY OF THE AVIAN FAMILY FRINGILLIDAE: A TAXONOMIC STUDY ***
Except for the typographical correction noted below and a few minor changes
(missing/extra punctuation) which may have been made but not noted here, the
text is the same as presented in the original publication. Some text has
been rearranged to restore paragraphs that were split by tables or images.
Most of the illustrations have notation to denote the scale compared to the
original specimen (example: × 3). Due to the variation in monitor resolution
and geometry, the scale is most likely not correct; but is provided as a guide.
Page 187, Table 1 Item 5 : Intavenous => Intravenous
[Cover]
Museum of Natural History
Myology and Serology
of the Avian Family Fringillidae,
A Taxonomic Study
BY
WILLIAM B. STALLCUP
University of Kansas
Lawrence
1954
[Pg 157]
Museum of Natural History
Myology and Serology
of the Avian Family Fringillidae,
A Taxonomic Study
BY
WILLIAM B. STALLCUP
University of Kansas
Lawrence
1954
[Pg 158]
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard,
Robert W. Wilson
Volume 8, No. 2, pp. 157-211, figures 1-23, 4 tables
Published November 15, 1954
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1954
25-4632
[Pg 159]
of the Avian Family Fringillidae,
a Taxonomic Study
BY
WILLIAM B. STALLCUP
| PAGE | |
| Introduction | 160 |
| Myology of the Pelvic Appendage | 162 |
| General Statement | 162 |
| Materials and Methods | 163 |
| Description of Muscles | 164 |
| Discussion of Myological Investigations | 175 |
| Comparative Serology | 185 |
| General Statement | 185 |
| Preparation of Antigens | 186 |
| Preparation of Antisera | 188 |
| Methods of Serological Testing | 188 |
| Experimental Data | 190 |
| Discussion of Serological Investigations | 190 |
| Conclusions | 201 |
| Summary | 208 |
| Literature Cited | 210 |
[Pg 160]
The relationships of many groups of birds within the Order
Passeriformes are poorly understood. Most ornithologists agree
that some of the passerine families of current classifications are
artificial groups. These artificial groupings are the result of early
work which gave chief attention to readily adaptive external structures.
The size and shape of the bill, for example, have been
over-emphasized in the past as taxonomic characters. It is now
recognized that the bill is a highly adaptive structure and that it
frequently shows convergence and parallelism.
Since studies of external morphology have failed in some cases
to provide a clear understanding of the relationships of passerine
birds, it seems appropriate that attention be given to other morphological
features, to physiological features, and to life history studies
in an attempt to find other clues to relationships at the family and
subfamily levels.
This paper reports the results of a study of the relationships of
some birds of the Family Fringillidae and is based on the comparative
myology of the pelvic appendage and on the comparative
serology of saline-soluble proteins. Where necessary for comparative
purposes, birds from other families have been included in these
investigations.
It has long been recognized that the Fringillidae include dissimilar
groups. Recent work by Beecher (1951b, 1953) on the
musculature of the jaw and by Tordoff (1954) primarily on the
structure of the bony palate has emphasized the artificial nature
of the assemblage although these authors disagree regarding major
divisions within it (see below).
The Fringillidae have been distinguished from other families of
nine-primaried oscines by only one character—a heavy and conical
bill (for crushing seeds). Bills of this form have been developed
independently in several other, unrelated, groups; as Tordoff
(1954:7) has pointed out, Molothrus of the Family Icteridae,
Psittorostra of the Family Drepaniidae, and most members of the
Family Ploceidae have bills as heavy and conical as those of the
fringillids. The ploceids are distinguished from the fringillids by
a single external character: a fairly well-developed tenth primary
whereas in fringillids the tenth primary is absent or vestigial. Tordoff
(1954:20) points out, however, that this distinction is of limited
value since in other passerine families the tenth primary may be
present in some species of a genus and absent in others. The Genus
[Pg 161]
Vireo is an example. Furthermore, at least one ploceid (Philetairus)
has a small, vestigial tenth primary, whereas some fringillids
(Emberizoides, for example) possess a tenth primary which is
rather large and ventrally placed (Chapin, 1917:253-254). Thus,
it is obvious that studies based on other features are necessary in
order to attain a better understanding of the relationships of the
birds involved.
Sushkin's studies (1924, 1925) of the structure of the bony and
horny palates have served as a basis for the division of the Fringillidae
into as many as five subfamilies (Hellmayr, 1938:v): Richmondeninae,
Geospizinae, Fringillinae, Carduelinae, and Emberizinae.
Beecher (1951b:280) points out that "the richmondenine finches
arise so uninterruptedly out of the tanagers that ornithologists have
had to draw the dividing line between the two groups arbitrarily."
His study of pattern of jaw-musculature substantiates this. He
states further that the cardueline finches arise without disjunction
from the tanagers. He suggests, therefore, that the two groups of
"tanager-finches" be made subfamilies of the Thraupidae and that
a third subfamily be maintained for the more typical tanagers. He
states that the emberizine finches are of different origin, arising from
the wood warblers (1953:307). Beecher (1951a:431; 1953:309)
includes the Dickcissel, Spiza americana, in the Family Icteridae,
chiefly on the basis of jaw muscle-pattern and the horny palate.
Tordoff (1954:10-11) presents evidence that the occurrence of
palato-maxillary bones in nine-primaried birds indicates relationship
among the forms possessing them. He points out that all fringillids
except the Carduelinae possess palato-maxillaries that are either
free or more or less fused to the prepalatine bar. He points out also
that in all carduelines, the prepalatine bar is flared at its juncture
with the premaxilla, and that the mediopalatine processes are fused
across the midline; noncardueline fringillids lack these characteristics.
In addition to the above he cites differences between the
carduelines and the "other" fringillids in the appendicular skeletons,
in geographic distribution, in patterns of migration, and in habits.
Tordoff concludes, therefore, that the carduelines are not fringillids
but ploceids, their closest affinities being with the ploceid Subfamily
Estrildinae. On the basis of palatal structure, the Fringillinae and
Geospizinae are combined with the Emberizinae, the name Fringillinae
being maintained for the subfamily. The tanagers merge with
the Richmondeninae on the one hand and with the Fringillinae on
the other. On this basis, Tordoff (1954:32) suggests that the Family
[Pg 162]
Fringillidae be divided into subfamilies as follows: Richmondeninae,
Thraupinae, and Fringillinae. The carduelines are placed as
the Subfamily Carduelinae in the Family Ploceidae.
From the foregoing, it is apparent that the two most recent lines
of research have given rise to conflicting theories regarding relationships
within the Family Fringillidae. The purpose of my investigation,
therefore, has been to gather information, from other fields,
which might clarify the relationships of these birds.
Since the muscle pattern of the leg in the Order Passeriformes is
thought to be one of long standing and slow change, any variation
which consistently distinguishes one group of species from another
could be significant. With the hope that such variation might be
found, a study of the comparative myology of the legs was undertaken.
The usefulness of comparative serology as a means of determining
relationship has been demonstrated in many investigations. Its use
in this instance was undertaken for several reasons: comparative
serology has its basis in biochemical systems which seem to evolve
slowly; its methods are objective; and its use has, heretofore, resulted
in the accumulation of data which seem compatible, in most
instances, with data obtained from other sources.
I acknowledge with pleasure the guidance received in this study
from Prof. Harrison B. Tordoff of the University of Kansas. I am
indebted also to Prof. Charles A. Leone without whose direction
and assistance the serological investigations would not have been
possible; to Professors E. Raymond Hall and A. Byron Leonard
whose suggestions and criticisms have been most helpful in the
preparation of this paper; and to T. D. Burleigh of the U. S. Fish
and Wildlife Service for gifts of several specimens used in this work.
Assistance with certain parts of the study were received from a contract
(NR163014) between the Office of Naval Research of the
United States Navy and the University of Kansas.
In an excellent paper in which the muscles of the pelvic appendage
of birds are carefully and accurately described, Hudson (1937)
reviewed briefly the more important literature pertaining to the
musculature of the leg which had been published to that date. A
review of such information here, therefore, seems unnecessary.
Myological formulae suggested by Garrod (1873, 1874) have
[Pg 163]
been extensively used by taxonomists as aids in characterizing the
orders of birds. Relatively few investigations, however, involving
the comparative myology of the leg have been undertaken at family
and subfamily levels. The works of Fisher (1946), Hudson (1948),
and Berger (1952) are notable exceptions.
The terminology for the muscles used in this paper follows that
of Hudson (1937), except that I have followed Berger (1952) in
Latinizing all names. Homologies are not given since these are
reviewed by Hudson. Osteological terms are from Howard (1929).
Specimens were preserved in a solution of one part formalin to eight parts
of water. Thorough injection of all tissues was necessary for satisfactory preservation.
Most of the down and contour feathers were removed to allow the
preservative to reach the skin.
In preparing specimens for study, the legs and pelvic girdle were removed
and washed in running water for several hours to remove much of the formalin.
They were then transferred to a mixture of 50 per cent alcohol and a small
amount of glycerine.
All specimens were dissected with the aid of a low power binocular microscope.
Where possible, several specimens of each species were examined for
individual differences. Such differences were found to be slight, involving
mainly size and shape of the muscles. The size is dependent partly on the
age of the bird, muscles from older birds being larger and better developed.
The shape of a muscle (whether long and slender or short and thick) is due in
part to the position in which the leg was preserved; that is to say, a muscle
may be extended in one bird and contracted in another. For these reasons,
descriptions and comparisons are based mainly on the origin and insertion of a
muscle and on its position in relation to adjoining muscles.
Birds dissected in this study are listed below (in the order of the A. O. U.
Check-List):
SPECIES
Vireo olivaceus (Linnaeus) Seiurus motacilla (Vieillot) Passer domesticus (Linnaeus) Estrilda amandava (Linnaeus) Poephila guttata (Reichenbach) Icterus galbula (Linnaeus) Molothrus ater (Boddaert) Piranga rubra (Linnaeus) Richmondena cardinalis (Linnaeus) Guiraca caerulea (Linnaeus) Passerina cyanea (Linnaeus) Spiza americana (Gmelin) Hesperiphona vespertina (Cooper) Carpodacus purpureus (Gmelin) | Pinicola enucleator (Linnaeus) Leucosticte tephrocotis (Swainson) Spinus tristis (Linnaeus) Loxia curvirostra Linnaeus Chlorura chlorura (Audubon) Pipilo erythrophthalmus (Linnaeus) Calamospiza melanocorys Stejneger Chondestes grammacus (Say) Junco hyemalis (Linnaeus) Spizella arborea (Wilson) Zonotrichia querula (Nuttall) Passerella iliaca (Merrem) Calcarius lapponicus (Linnaeus) |
[Pg 164]
The descriptions which follow are those of the muscles in the leg of the
Red-eyed Towhee, Pipilo erythrophthalmus. Differences between species,
where present, are noted for each muscle. The term thigh is used to refer to
the proximal segment of the leg; the term crus is used for that segment of the
leg immediately distal to the thigh.
Musculus iliotrochantericus posticus (Fig. 2).—The origin of this muscle is
fleshy from the entire concave lateral surface of the ilium anterior to the acetabulum.
The fibers converge posteriorly, and the muscle inserts by a short,
broad tendon on the lateral surface of the femur immediately distal to the
trochanter. It is the largest muscle which passes from the ilium to the femur.
Action.—Moves femur forward and rotates it anteriorly.
Comparison.—No significant differences noted among the species studied.
Musculus iliotrochantericus anticus (Fig. 3).—Covered laterally by the m.
iliotrochantericus posticus, this slender muscle has a fleshy origin from the
anteroventral edge of the ilium between the origins of the m. sartorius anteriorly
and the m. iliotrochantericus medius posteriorly. The m. iliotrochantericus
anticus is directed caudoventrally and inserts by a broad, flat tendon on the
anterolateral surface of the femur between the heads of the m. femorotibialis
externus and m. femorotibialis medius and just distal to the insertion of the m.
iliotrochantericus medius.
Action.—Moves femur forward and rotates it anteriorly.
Comparison.—No significant differences noted among the species studied.
Musculus iliotrochantericus medius (Fig. 3).—Smallest of the three iliotrochantericus
muscles, this bandlike muscle has a fleshy origin from the ventral
edge of the ilium just posterior to the origin of the m. iliotrochantericus anticus.
The fibers are directed caudoventrally, and the insertion is tendinous on the
anterolateral surface of the femur between the insertion of the other two iliotrochantericus
muscles.
Action.—Moves femur forward and rotates it anteriorly.
Comparison.—No significant differences noted among the species studied.
Musculus iliacus (Figs. 4, 5).—Arising from a fleshy origin on the ventral
edge of the ilium just posterior to the origin of the m. iliotrochantericus medius,
this small slender muscle passes posteroventrally to its fleshy insertion on the
posteromedial surface of the femur just proximal to the origin of the m. femorotibialis
internus.
Action.—Moves femur forward and rotates it posteriorly.
Comparison.—No significant differences among the species studied.
Musculus sartorius (Figs. 1, 4).—A long, straplike muscle, the sartorius
forms the anterior edge of the thigh. The origin is fleshy, half from the
anterior edge of the ilium and from the median dorsal ridge of this bone and
half from the posterior one or two free dorsal vertebrae. The insertion is
fleshy along a narrow line on the anteromedial edge of the head of the tibia and
on the medial region of the patellar tendon.
Action.—Moves thigh forward and upward and extends shank.
Comparison.—In Loxia and Spinus, only one-third of the origin is from the
last free dorsal vertebra. In Hesperiphona, Carpodacus, Pinicola, and Leucosticte,
only one-fifth of the origin is from this vertebra.
[Pg 165]
Musculus iliotibialis (Fig. 1).—Broad and triangular, this muscle covers
most of the deeper muscles of the lateral aspect of the thigh. The middle
region is fused with the underlying femorotibialis muscles. In the distal half
of this muscle there are three distinct parts; the anterior and posterior edges
are fleshy and the central part is aponeurotic. The origin is from a narrow line
along the iliac crests—from the origin of the m. sartorius, anteriorly, to the
origin of the m. semitendinosus posteriorly. The origin is aponeurotic in the
preacetabular region but fleshy in the postacetabular region. The distal part
of the muscle is aponeurotic and joins with the femorotibialis muscles in the
formation of the patellar tendon. This tendon incloses the patella and inserts
on a line along the proximal edges of the cnemial crests of the tibiotarsus.
Action.—Extends crus.
Comparison.—In Vireo the central aponeurotic portion of this muscle is
absent.
Musculus femorotibialis externus (Fig. 2).—Covering the lateral and anterolateral
surfaces of the femur, this large muscle has a fleshy origin from the
lateral edge of the proximal three-fourths of the femur. The origin separates
the insertion of the m. iliotrochantericus anticus from that of the m. ischiofemoralis
and, in turn, is separated from the origin of the m. femorotibialis
medius by the insertions of the m. iliotrochantericus anticus and m. iliotrochantericus
medius. Approximately midway of the length of the femur this
muscle fuses anteromesially with the m. femorotibialis medius. Distally, the
m. femorotibialis externus contributes to the formation of the patellar tendon
which inserts on a line along the proximal edges of the cnemial crests of the
tibiotarsus.
Action.—Extends crus.
Comparison.—No significant differences noted among the species studied.
Musculus femorotibialis medius (Figs. 2, 4).—The origin of this muscle,
which lies along the anterior edge of the femur, is fleshy from the entire length
of the femur proximal to the level of attachment of the proximal arm of the
biceps loop. Laterally this muscle is completely fused for most of its length
with the m. femorotibialis externus and contributes to the formation of the
patellar tendon, which inserts on a line along the proximal edges of the cnemial
crests of the tibiotarsus. Many of the fibers, nevertheless, insert on the proximal
edge of the patella.
Action.—Extends crus.
Comparison.—No significant differences noted among the species studied.
Musculus femorotibialis internus (Fig. 4).—One of the most superficial
muscles lying on the medial surface of the thigh, this muscle is divided,
especially near the distal end, into two parts, lateral and medial. The origin of
the lateral part is fleshy from a line on the medial surface of the femur; the
origin begins proximally at a point near the insertion of the m. iliacus. The
medial, bulkier part of the muscle has a fleshy origin on the medial surface of
the lower one-third of the femur. The two parts fuse to some extent above the
points of insertion and insert on the medial edge of the head of the tibia.
Action.—Rotates tibia anteriorly.
Comparison.—Two parts of this muscle variously fused; otherwise, no significant
differences in the species studied.
Musculus piriformis (Fig. 3).—This muscle is represented by the pars caudifemoralis
[Pg 166]
only, the pars iliofemoralis being absent in passerine birds as far as
is known. The pars caudifemoralis is flat, somewhat spindle-shaped, and passes
anteroventrally from the pygostyle to the femur. The origin is tendinous from
the anteroventral edge of the pygostyle, and the insertion is semitendinous on
the posterolateral surface of the shaft of the femur about one-fourth its length
from the proximal end.
Action.—Moves femur posteriorly and rotates it in this direction; moves tail
laterally and depresses it.
Comparison.—No significant differences noted among the species studied.
Musculus semitendinosus (Figs. 2, 3, 5).—The origin from the extreme posterior
edge of the posterior iliac crest of the ilium is fleshy and is aponeurotic
from the last vertebra of the synsacrum and the transverse processes of several
caudal vertebrae. The straplike belly passes along the posterolateral margin
of the thigh. Immediately posterior to the knee, the muscle is divided transversely
by a ligament. That portion passing anteriorly from the ligament is
the m. accessorius semitendinosi (here considered a part of the m. semitendinosus)
and is discussed below. The ligament continues distally in two parts;
one part inserts on the medial surface of the pars media of the m. gastrocnemius
and the other part fuses with the tendon of insertion of the m. semimembranosus.
The m. accessorius semitendinosi extends anteriorly from the above mentioned
ligament to a fleshy insertion on the posterolateral surface of the femur
immediately proximal to the condyles.
Action.—Moves femur posteriorly, flexes the crus and aids in extending the
tarsometatarsus.
Comparison.—No significant differences noted among the species studied.
Musculus semimembranosus (Figs. 3, 4, 5).—This straplike muscle passes
along the posteromedial surface of the thigh. The origin is semitendinous along
a line on the ischium, from a point dorsal to the middle of the ischiopubic
fenestra to the posterior end of the ischium, and from a small area of the
abdominal musculature posterior to the ischium. The insertion is by means of
a broad, thin tendon on a ridge on the medial surface of the tibia immediately
distal to the head of this bone. The tendon of insertion passes between the
head of the pars media and pars interna of the m. gastrocnemius and is fused
with the tendon of the m. semitendinosus.
Action.—Flexes crus.
Comparison.—No significant differences noted among the species studied.
Musculus biceps femoris (Fig. 2).—Long, thin, and somewhat triangular,
this muscle lies on the lateral side of the thigh just underneath the m. iliotibialis.
Its origin is from a line along the anterior and posterior iliac crests underneath
the origin of the m. iliotibialis. Anterior to the acetabulum the origin is aponeurotic,
and the edge of this aponeurosis passes over the proximal end of the
femur. The origin posterior to the acetabulum is fleshy. The most anterior
point of origin is difficult to ascertain but it lies near the center of the anterior
iliac crest. The most posterior point of origin is immediately dorsal to the
posterior end of the ilioischiatic fenestra. Behind the knee the fibers of this
muscle converge to form the strong tendon of insertion which passes through
the biceps loop, under the tendon of origin of the m. flexor perforatus digiti II,
[Pg 167]
and inserts on a small tubercle on the posterolateral edge of the fibula at the
point of the tibia-fibula fusion.
The biceps loop is tendinous and the distal end attaches to a protuberance
on the posterolateral edge of the femur at the proximal edge of the external
condyle. The proximal end attaches to the anterolateral edge of the femur immediately
proximal to the distal end of the loop, which extends posterior to the
femur. The distal arm of this loop is connected with the tendon of origin of
the m. flexor perforatus digiti II by a strong tendon.
Action.—Flexes crus.
Comparison.—No significant differences noted among the species studied.
Musculus ischiofemoralis (Fig. 3).—Short and thick, this muscle arises directly
from the lateral surface of the ischium between the posterior iliac crest
and the ischiopubic fenestra. The area of origin extends to the posterior edge
of the ischium. The insertion is tendinous on the lateral surface of the trochanter
opposite the insertion of the m. iliotrochantericus medius.
Action.—Moves femur posteriorly and rotates it in this direction.
Comparison.—No significant differences noted among the species studied.
Musculus obturator internus (Figs. 4, 7).—Lying on the inside of the pelvis
and covering the medial surface of the ischiopubic fenestra, is this flat, pinnate,
leaf-shaped muscle. The origin is fleshy and is from the ischium and pubis
around the edges of this fenestra; none of the fibers arises from the membrane
stretched across the fenestra. Anteriorly the fibers converge and form a strong
tendon that passes through the obturator foramen and inserts on the posterolateral
surface of the trochanter of the femur.
Action.—Rotates femur posteriorly.
Comparison.—No significant differences noted among the species studied.
Musculus obturator externus (Fig. 7).—Short and fleshy, this muscle consists
of two parts which are not easily separable but which may be traced throughout
its length. The parts are more nearly distinct at the origin. The dorsal
part arises directly from the ischium along the dorsal edge of the obturator
foramen. The larger ventral part arises directly from the anterior and ventral
edges of the obturator foramen. The fibers of the dorsal part pass anteriorly,
cover the tendon of the m. obturator internus laterally, and insert on the trochanter
around the point of insertion of the latter muscle. The fibers of the
ventral part pass parallel with the tendon of the m. obturator internus and insert
on the trochanter immediately distal and posterior to the tendon of the latter
muscle.
Action.—Rotates femur posteriorly.
Comparison.—In Passer, Estrilda, Poephila, Hesperiphona, Carpodacus, Pinicola,
Leucosticte, Spinus and Loxia, this muscle is undivided and, in its position,
origin, and insertion, resembles the ventral part of the bipartite muscle
described above. The origin is from the anterior and ventral edges of the
obturator foramen and the insertion is on the trochanter of the femur immediately
distal and posterior to the insertion of the m. obturator internus. In all
other genera examined, the muscle is bipartite. In Chlorura the dorsal part is
larger and better developed than it is in the other genera.
Musculus adductor longus et brevis (Figs. 3, 4, 5).—Consisting of two distinct,
straplike parts, this large muscle lies on the medial surface of the thigh,
posterior to the femur.
[Pg 168]
The pars anticus has a semitendinous origin on a line that extends posteriorly
from the posteroventral edge of the obturator foramen to a point half way across
the membrane that covers the ischiopubic fenestra. The insertion is fleshy
along the posterior surface of the femur from the level of the insertion of the
m. piriformis distally to the medial surface of the internal condyle.
The pars posticus originates by a broad, flat tendon on a line across the
posterior half of the membrane that covers the ischiopubic fenestra. The insertion
is at the point of origin of the pars media of the m. gastrocnemius on
the posteromedial surface of the proximal end of the internal condyle of the
femur. There is a broad tendinous connection with the proximal end of the
pars media of the m. gastrocnemius. The anterior edge of the pars posticus is
overlapped medially by the posterior edge of the pars anticus.
Action.—Flexes thigh; may flex crus also and may extend tarsometatarsus.
Comparison.—In Vireo olivaceous, the origin of this muscle does not extend
the length of the ischiopubic fenestra. The origin, furthermore, is along the
dorsal edge of the ischiopubic fenestra and not from the membrane covering
the fenestra. Finally, in this species, the origin of the pars posticus is fleshy.
Musculus tibialis anticus (Figs. 2, 5).—Lying along the anterior edge of the
crus, a part of this muscle is covered by the m. peroneus longus. The origin is
by two distinct heads, each of which is pinnate. The anterior head arises
directly from the edges of the outer and inner cnemial crests. The posterior
head arises by a short, strong tendon from a small pit on the anterodistal edge
of the external condyle of the femur. This tendon and the proximal end of
the muscle pass between the head of the fibula and the outer cnemial crest.
The two heads of the muscle fuse at a place slightly more than one-half of the
distance down the crus. At the distal end of the crus this muscle gives rise to
a strong tendon which passes under a fibrous loop immediately proximal to
the external condyle in company with the m. extensor digitorum longus and
which passes between the condyles of the tibia and inserts on a tubercle on the
anteromedial edge of the proximal end of the tarsometatarsus.
Action.—Flexes tarsometatarsus.
Comparison.—No significant differences noted among the species studied.
Musculus extensor digitorum longus (Figs. 3, 5, 8).—Slender and pinnate,
this muscle lies along the anteromedial surface of the tibia. The origin is fleshy
from most of the region between the cnemial crests and from a line along the
anterior surface of the proximal fourth of the tibia. Approximately two-thirds
of the distance down the crus the muscle gives rise to the tendon of insertion
which passes through the fibrous loop near the distal end of the tibia in company
with the m. tibialis anticus. The tendon then passes along beneath the
supratendinal bridge at the distal end of the tibia, traverses the anterior intercondylar
fossa, and passes beneath a bony bridge on the anteromedial surface
of the proximal end of the tarsometatarsus. The tendon continues along the
anterior surface of the tarsometatarsus to a point immediately above the bases
of the toes and there gives rise to three branches, one to the anterior surface of
each foretoe. The insertions of each branch are on the anterior surfaces of the
phalanges as shown in Fig. 8.
Action.—Extends foretoes.
Comparison.—This muscle is weakly developed in Leucosticte and Calvarius;
the belly is slender and extends only half way down the crus before giving rise
[Pg 169]
to the tendon of insertion. The functional significance of this variation is difficult
to understand. The convergence in muscle pattern shown by these two
genera, however, is in all probability the result of similarities in behavior patterns.
These birds perch less frequently than do the other birds studied. Thus,
the toes are neither flexed nor extended as often; the smaller size of the m.
extensor digitorum longus may have resulted in part from this lessened activity.
Except for the variations just noted, there are no significant differences among
the species studied; even the rather complex patterns of insertion are identical.
Musculus peroneus longus (Fig. 1).—Relatively thin and straplike, this
muscle lies on the anterolateral surface of the crus and is intimately attached
to the underlying muscles. The part of the origin from the proximal edges of
the inner and outer cnemial crests is semitendinous but the part of the origin
from the lateral edge of the shaft of the fibula is tendinous. Approximately
two-thirds the distance down the crus the muscle gives rise to the tendon of
insertion. Immediately above the external condyle of the tibiotarsus this tendon
divides. The posterior branch inserts on the proximal end of the lateral edge
of the tibial cartilage. The anterior branch passes over the lateral surface of
the external condyle to the posterior surface of the tarsometatarsus and there
unites with the tendon of the m. flexor perforatus digiti III.
Action.—Extends tarsometatarsus and flexes third digit.
Comparison.—No significant differences noted among the species studied.
Musculus peroneus brevis (Figs. 2, 3).—Lying along the anterolateral surface
of the tibia, this slender, pinnate muscle arises from a fleshy origin along
this surface and along the anterior surface of the fibula from a point immediately
proximal to the insertion of the m. biceps femoris to a point approximately
two-thirds of the way down the crus. Near the distal end of the tibia
the muscle gives rise to the tendon of insertion that passes through a groove on
the anterolateral edge of the tibia just above the external condyle. Here the
tendon is held in place by a broad fibrous loop and passes under the anterior
branch of the tendon of insertion of the m. peroneus longus and inserts on a
prominence on the lateral edge of the proximal end of the tarsometatarsus.
Action.—Extends tarsometatarsus and may abduct it slightly.
Comparison.—No significant differences noted among the species studied.
Musculus gastrocnemius (Figs. 1, 4).—The largest muscle of the pelvic appendage,
it covers superficially all of the posterior surface, most of the medial
surface, and half of the lateral surface of the crus. The muscle originates by
three distinct heads.
The pars externa covers the posterolateral surface of the crus, is intermediate
in size between the other two heads, and arises by a short, strong tendon from
a small bony protuberance on the posterolateral side of the distal end of the
femur immediately proximal to the fibular condyle. The tendon is intimately
connected with the distal arm of the loop for the m. biceps femoris.
The pars media is the smallest of the three heads and lies on the medial surface
of the crus. The head of the pars media is separated from the pars
interna by the tendon of insertion of the m. semimembranosus and originates
by a short, strong tendon from the posteromedial surface of the proximal end
of the internal condyle of the femur. The proximal portion of the pars media
has tendinous connections with the tendon of the m. semitendinosus and with
the pars posticus of the m. adductor longus et brevis.
[Pg 170]
The pars interna is the largest of the three heads and covers most of the
medial surface of the crus. This head in its proximal portion is distinctly
divided into anterior and posterior parts, the former overlapping the latter
medially. The origin of the posterior part is fleshy from the anterior half of
the tibial head. Some of the fibers of the anterior part arise directly from the
inner cnemial crest while its remaining fibers arise from the patellar tendon
(Fig. 1) and form a band that extends around the anterior surface of the knee,
covering the insertion of the m. sartorius.
Approximately half way down the crus, the three heads give rise to the
tendon of insertion, the tendo achillis, which passes over and is tightly bound
to the posterior surface of the tibial cartilage. The insertion is tendinous on
the posterior surface of the hypotarsus and along the posterolateral ridge of
the tarsometatarsus. This tendon seems to be continuous with a fascia which
forms a sheath around the posterior surface of the tarsometatarsus holding the
other tendons of this region firmly in the posterior sulcus.
Action.—Extends tarsometatarsus.
Comparison.—Study of the pars externa and pars media reveals no significant
differences among the species dissected. The pars interna, however, is
subject to some variation which is described below.
Pars interna bipartite
Vireo Seiurus Icterus Molothrus Piranga Richmondena Guiraca Passerina Spiza | Chlorura Pipilo Calamospiza Chondestes Junco Spizella Zonotrichia Passerella Calcarius |
The two parts of the m. gastrocnemius are most distinct in Vireo. Icterus,
Molothrus, Richmondena, Guiraca, and Passerina lack the fibrous band that
passes around the front of the knee. In Spiza this band of fibers is smaller
than in the other species.
Pars interna undivided
Passer Estrilda Poephila Hesperiphona Carpodacus | Pinicola Leucosticte Spinus Loxia |
In Leucosticte, although the pars interna is undivided, there is a band of
fibers which extends around the front of the knee (see discussion, p. 183).
Musculus plantaris (Fig. 5).—Small and slender, this muscle lies on the
posteromedial surface of the crus, beneath the pars interna of the m. gastrocnemius
and originates by fleshy fibers from the posteromedial surface of the
proximal end of the tibia immediately distal to the internal articular surface.
The belly extends approximately one-sixth of the way down the crus and gives
rise to a long, slender tendon that inserts on the proximomedial edge of the
tibial cartilage.[Pg 171]
Action.—Extends tarsometatarsus.
Comparison.—No significant differences noted among the species studied.
Musculus flexor perforatus digiti II (Figs. 3, 9).—This is a slender muscle
which lies on the lateral side of the crus beneath the pars externa of the m.
gastrocnemius and is intimately connected anteromedially with the m. flexor
digitorum longus and posteromedially with the m. flexor hallucis longus. The
origin is by a strong tendon from the lateral surface of the external condyle of
the femur at the point of origin of the m. flexor perforans et perforatus digiti II.
This tendon serves also as the origin of the anterior head of the m. flexor
hallucis longus. The tendon connects also by a broad tendinous band with the
distal arm of the loop for the m. biceps femoris and by a similar band with the
lateral edge of the fibula immediately distal to the head. The tendon of insertion
passes distally, perforates the tibial cartilage near its lateral edge, traverses
the middle medial canal of the hypotarsus (Fig. 6), and passes distally
to the foot. At the distal end of the tarsometatarsus the tendon is held against
the medial surface of the first metatarsal by a straplike sheath. The tendon
then passes over a sesamoid bone between the first metatarsal and the base of
the second digit and is bound to this bone by a sheath. The tendon inserts
mainly along the posteromedial edge of the proximal end of the first phalanx
of the second digit, although the termination is sheathlike and covers the entire
posterior surface of this phalanx. This sheathlike termination is perforated by
the tendons of the m. flexor perforans et perforatus digiti II and the branch of
the m. flexor digitorum longus that inserts on the second digit.
Action.—Flexes second digit.
Comparison.—In Vireo this muscle is larger and more deeply situated than
it is in the other species examined and has no connection with the m. flexor
hallucis longus.
Musculus flexor perforatus digiti III (Fig. 5).—Long and flattened, this
muscle lies on the posteromedial side of the crus beneath the m. gastrocnemius.
The belly is tightly fused laterally with the belly of the m. flexor hallucis longus
and posteriorly with the belly of the m. flexor perforatus digiti IV. The origin
is by a long, strong tendon from a small tubercle just medial to, and at the
proximal end of, the external condyle of the femur. Below the middle of the
crus this muscle terminates in a strong tendon which perforates the tibial
cartilage near its lateral edge. In this region the tendon is sheathlike and
wrapped around the tendon of the m. flexor perforatus digiti IV. These two
tendons together pass through the posterolateral canal of the hypotarsus (Fig.
6). Immediately distal to the hypotarsus the two tendons separate, and the
tendon of the m. flexor perforatus digiti III receives a branch of the tendon of
the m. peroneus longus. The tendon passes distally over the surface of the
second trochlea, and its insertion is sheathlike on the posterior surface of the
first phalanx, and on the proximal end of the second. In the area of insertion
this tendon is perforated by that of the m. flexor perforans et perforatus digiti
III and by that of the m. flexor digitorum longus to the third digit.
Action.—Flexes digit III.
Comparison.—In Passer, Estrilda, Poephila, Hesperiphona, Carpodacus,
Pinicola, Leucosticte, Spinus, and Loxia the edges of the sheathlike tendon are
thickened at the points of insertion, so that the tendon appears to have two
branches which insert along the posterolateral edges of the first phalanx and are
connected medially by a fascia.
[Pg 172]
Musculus flexor perforatus digiti IV (Fig. 3).—Extending along the posterior
edge of the crus, this slender muscle lies beneath the m. gastrocnemius.
The belly is fused with those of the m. flexor hallucis longus and m. flexor perforatus
digiti III. Its origin is fleshy from the intercondyloid region of the distal
end of the femur and has a few fibers arising from the tendon of origin of the
m. flexor perforatus digiti III. Near the distal end of the crus the muscle gives
rise to the strong tendon of insertion which perforates the tibial cartilage near
its lateral edge and in this region is ensheathed by the tendon of the m. flexor
perforatus digiti III. The two tendons pass together through the posterolateral
canal of the hypotarsus (Fig. 6). The tendon continues distally along the
tarsometatarsus and the posterior surface of digit IV. The tendon bifurcates
at approximately the middle of the first phalanx. A short lateral branch inserts
on the posterolateral edge of the proximal end of the second phalanx. The
long medial branch is perforated by a branch of the m. flexor digitorum longus;
the distal end is flattened, has thickened edges, and inserts over the posterior
surfaces of the distal end of the second phalanx, and over the proximal end of
the third phalanx.
Action.—Flexes digit IV.
Comparison.—No significant differences noted among the species studied.
Musculus flexor perforans et perforatus digiti II (Figs. 2, 9).—Small and
spindle-shaped, this muscle lies on the posterolateral side of the crus immediately
beneath the pars externa of the m. gastrocnemius. The origin is fleshy
and arises in company with the m. flexor perforans et perforatus digiti III from
a point on the posterolateral surface of the distal end of the femur between the
point of origin of the pars externa of the m. gastrocnemius and the fibular
condyle. The belly extends approximately one-fourth of the way down the
crus and gives rise to the tendon of insertion which passes distally and superficially
through the posterior edge of the tibial cartilage. The tendon traverses
the posteromedial canal of the hypotarsus (Fig. 6) and continues along the
posterior surface of the tarsometatarsus. Between the first metatarsal and the
base of the second digit the tendon is enclosed by the medial surface of a
sesamoid bone. This tendon then perforates that of the m. flexor perforatus
digiti II at the level of the first phalanx and in turn is perforated by the tendon
of the m. flexor digitorum longus at the proximal end of the second phalanx.
The insertion is on the posterior surface of the second phalanx.
Action.—Flexes digit II.
Comparison.—In Passer, Estrilda, Poephila, Hesperiphona, Carpodacus,
Pinicola, Leucosticte, Spinus, and Loxia the proximal portion of this muscle is
more intimately connected with the posterior edge of the m. flexor perforans et
perforatus digiti III than it is in the other species examined.
Musculus flexor perforans et perforatus digiti III (Fig. 2).—Long and pinnate,
this muscle lies on the lateral surface of the crus beneath the m. peroneus
longus and pars externa of the m. gastrocnemius. There are two distinct heads.
The origin of the anterior head is fleshy from the proximal edge of the outer
cnemial crest and from the internal edge of the distal end of the patellar tendon.
The posterior head arises by a tendon from the femur in company with the m.
flexor perforans et perforatus digiti II, is connected also with the tendon of
origin of the m. flexor perforatus digiti II, and is loosely attached to the head
of the fibula. Fibers from the belly of the muscle attach throughout its length
[Pg 173]
to the lateral edge of the fibula, and the muscle is tightly fused also with
adjacent muscles. The tendon of insertion is formed approximately one-half the
way down the crus. The tendon perforates the posterior surface of the tibial
cartilage and passes through the posteromedial canal of the hypotarsus (Fig.
6). At the base of the third digit the tendon ensheathes that of the m. flexor
digitorum longus and the two together perforate the tendon of the m. flexor
perforatus digiti III. Immediately distal to this perforation the tendon of the
m. flexor perforans et perforatus digiti III ceases to ensheath that of the m.
flexor digitorum longus. The latter passes beneath that of the former. Near
the distal end of the second phalanx the tendon of the m. flexor digitorum
longus perforates that of the m. flexor perforans et perforatus digiti III. The
latter inserts on the posterior surface of the distal end of the second phalanx and
the proximal end of the third.
Action.—Flexes digit III.
Comparison.—In Passer, Estrilda, and Poephila, and in all the cardueline
finches examined the proximal portion of this muscle is more intimately connected
with the anterior edge of the m. flexor perforans et perforatus digiti II
than it is in the other species examined.
Musculus flexor digitorum longus (Figs. 3, 5).—This strong, pinnate muscle
is deeply situated along the posterior surfaces of the tibia and fibula. There
are two distinct heads of origin. The lateral head arises by means of fleshy
fibers from the posterior edge of the head of the fibula. The medial head arises
by means of fleshy fibers from the region under the ledgelike external and internal
articular surfaces of the proximal end of the tibia. Neither head has any
connection with the femur in contrast to the condition, described by Hudson
(1937: 46-47) in the crow, Corvus brachyrhynchos, and in the raven, Corvus
corax. Near the point of insertion of the m. biceps femoris the two heads fuse.
The common belly is attached by fleshy fibers to the posterior surface of the
tibia and fibula for two-thirds of the distance down the crus. Near the distal
end of the crus the muscle terminates in a strong tendon which passes deeply
through the tibial cartilage and traverses the anteromedial canal of the hypotarsus
(Fig. 6). About midway down the tarsometatarsus this tendon becomes
ossified. Immediately above the bases of the toes it gives rise to three branches,
one to the posterior surface of each of the foretoes. These branches perforate
the other flexor muscles of the toes as described in the accounts of those muscles
and insert as follows: The branch to digit II inserts on the base of the ungual
phalanx and by a stout, tendinous slip on the distal end of the second phalanx
(Fig. 9). The branch to digit III inserts on the base of the distal end of the
third phalanx and a stronger slip to the distal end of the second or proximal end
of the third. The branch to digit IV inserts on the base of the ungual phalanx,
with one tendinous slip to the distal end of the third phalanx and another to
the distal end of the fourth.
Action.—Flexes foretoes.
Comparison.—No significant differences noted among the species studied.
Musculus flexor hallucis longus (Fig. 3).—Situated immediately posterior to
the m. flexor digitorum longus, the belly of this large, pinnate muscle is intimately
connected anteriorly to that of the m. flexor perforatus digiti II. The
m. flexor hallucis longus arises by two heads which are separated by the tendon
of insertion of the m. biceps femoris. The smaller anterior head arises from
[Pg 174]
the same tendon as does the m. flexor perforatus digiti II. The larger posterior
head arises by means of fleshy fibers from the intercondyloid region of the posterior
surface of the femur along with the m. flexor perforatus digiti III and IV.
The two heads join just distal to the point of insertion of the m. biceps femoris.
There is no trace of a tendinous band connecting the two heads as there is in
the crow and in the raven (Hudson, 1937:49). Near the distal end of the
shank the muscle gives rise to a strong tendon which perforates the tibial
cartilage along its lateral edge and passes through the anterolateral canal of
the hypotarsus (Fig. 6). The tendon crosses over to the medial surface of the
tarsometatarsus, passes distally, and perforates the sheathlike tendon of the m.
flexor hallucis brevis between the first metatarsal and the trochlea for digit II.
The tendon continues along the posterior surface of the hallux and has a
double insertion; the main tendon attaches to the base of the ungual phalanx
and a smaller branch inserts on the distal end of the proximal phalanx.
Action.—Flexes hallux.
Comparison.—In Vireo this muscle has only the posterior head of origin and
is not connected with the m. flexor perforatus digiti II. The muscle is proportionately
smaller and weaker than in any of the other species studied.
Musculus extensor hallucis longus (Fig. 4).—One of the smallest muscles of
the leg, the origin is fleshy from the anteromedial edge of the proximal end of
the tarsometatarsus. The belly is long and slender and terminates distally in
a slender tendon which passes distally along the posterior surfaces of the first
metatarsal and the first digit. The insertion is on the base of the ungual
phalanx. Near the distal end of the proximal phalanx, the tendon passes between
two thick bands of fibro-elastic tissue which insert also on the ungual
phalanx. These bands of tissue function as automatic extensors of the claw.
Action.—Extends hallux; action must be slight.
Comparison.—In Vireo this muscle is proportionately larger and better developed
than it is in any of the other species examined.
Musculus flexor hallucis brevis (Fig. 4).—This minute muscle has a fleshy
origin from the medial surface of the hypotarsus. The short belly terminates
in a weak, slender tendon which passes down the posteromedial surface of the
tarsometatarsus and into the space between the first metatarsal and the trochlea
for digit II. In this region the tendon envelops the tendon of the m. flexor
hallucis longus and inserts on the distal end of the first metatarsal and on the
proximal end of the first phalanx of the first digit.
Action.—Flexes hallux; action must be slight.
Comparison.—The small size of this muscle makes it exceedingly difficult to
study. The muscle is larger in Vireo than in any of the other species examined.
This may be correlated with the smaller size of the m. flexor hallucis longus in
this species. The muscle does not seem to be so well developed in the cardueline
finches as it is in the other species.
Musculus abductor digiti IV (Fig. 2).—Extremely small, delicate and difficult
to demonstrate, this muscle arises in a fleshy origin immediately from
underneath the posterior edge of the external cotyla of the tarsometatarsus. The
tendon of insertion is long and slender and inserts along the lateral edge of the
first phalanx of digit IV.
[Pg 175]
Action.—Abducts digit IV.
Comparison.—No significant differences noted among the species studied.
Musculus lumbricalis.—Semitendinous throughout its length, this muscle
arises from the ossified tendon of the m. flexor digitorum longus at a point immediately
proximal to the branching of this tendon. The insertion is on the
joint pulleys and capsules at the base of the third and fourth digits.
Action.—Hudson (1937:57) states that: "Meckel (vide Gadow—1891, p.
204) considered this muscle as serving to draw the joint pulley behind in order
to protect it from pinching during the bending of the toes. It perhaps also
tends to flex the third and fourth digits."
Comparison.—No significant differences noted among the species studied.
Simpson (1944:12) and others have emphasized that different
parts of organisms evolve at different rates. Beecher (1951b:275)
in stating that "... the hind limb is very similar in muscle
pattern throughout the Order Passeriformes and seems to have become
relatively static after attaining a high level of general efficiency
..." implies that the muscle pattern of the leg must be one of
long standing and slow change. This concept was emphasized by
Hudson (1937) who found but little variation in muscle pattern
among members of the several families of passerine birds. The concept
is further confirmed by the present investigation. The intricate
patterns of origin and of insertion seem to remain almost the same
throughout the order in spite of adaptive radiation which has occurred.
Two major differences in patterns of leg-musculature, however,
were found among the species studied, and these differences are
significant since they are consistent between subfamilies. The
muscles involved are the m. obturator externus and the pars interna
of the m. gastrocnemius.
The m. obturator externus is bipartite, consisting of dorsal and
ventral parts, in the passerine species studied by Hudson (1937) and
in all of the species examined by me except the ploceids and the
cardueline finches. In the ploceids and cardueline finches this
muscle is undivided and resembles in its position, origin, and insertion
only the ventral portion of the muscle found in the other birds
studied. It is difficult to imagine what advantage or disadvantage
might be associated with the bipartite or with the undivided condition.
The action of this muscle is to rotate the femur (right femur
clockwise, left femur counterclockwise), and certainly the greater
mass of the bipartite muscle could lend greater strength to such
action. The possible significance of this is discussed below.
[Pg 176]
Abd. dig. IV
Acc.
Add. long.
Anterolat. can. Anterolateral canal of hypotarsus
Anteromed. can. Anteromedial canal of hypotarsus
Bic. fem.
Bic. loop Loop for
Ext. cot. External cotyla
Ext. dig. l.
Ext. hal. l.
Fem. tib. ext.
Fem. tib. int.
Fem. tib. med.
F. dig. l.
F. hal. brev.
F. hal. l.
F. p. et p. d. II
F. p. et p. d. III
F. per. d. II
F. per. d. III
F. per. d. IV
Gas.
Iliacus
Il. tib.
Il. troc. ant.
Il. troc. med.
Il. troc. post.
Int. cot. Internal cotyla
Isch. fem.
Midmed. can. Midmedial canal of hypotarsus
Obt. ext.
Obt. int.
P. ant.
P. ext.
P. int.
P. med.
P. post.
Per. brev.
Per. long.
Pirif.
Plan.
Posterolat. can. Posterolateral canal of hypotarsus
Posteromed. can. Posteromedial canal of hypotarsus
Sar.
Semim.
Semit.
Tib. ant.
Tib. cart. Tibial cartilage
[Pg 177]
Fig. 1. Pipilo erythrophthalmus. Lateral view of the superficial muscles of the left leg, × 1.5.
[Pg 178]
Fig. 2. Pipilo erythrophthalmus. Lateral view of the left leg showing a deeper set of muscles. The superficial muscles iliotibialis, sartorius, gastrocnemius and peroneus longus have been removed, × 1.5.
[Pg 179]
Fig. 3. Pipilo erythrophthalmus. Lateral view of the left leg showing the still deeper muscles. In addition to those listed for figure 2, the following muscles have been wholly or partly removed: iliotrochantericus posticus, femorotibialis externus, femorotibialis medius, biceps femoris, semitendinosus, tibialis anticus, flexor perforans et perforatus digiti II, and flexor perforans et perforatus digiti III, × 1.5.
[Pg 180]
Fig. 4. Pipilo erythrophthalmus. Medial view of the superficial muscles of the left leg, × 1.5.
[Pg 181]
Fig. 5. Pipilo erythrophthalmus. Medial view of the left leg showing a deeper set of muscles than those seen in figure 4. The following superficial muscles have been removed: iliotibialis, sartorius, femorotibialis internus, obturator internus, adductor longus (pars posticus), gastrocnemius, and peroneus longus, × 1.5.
[Pg 182]
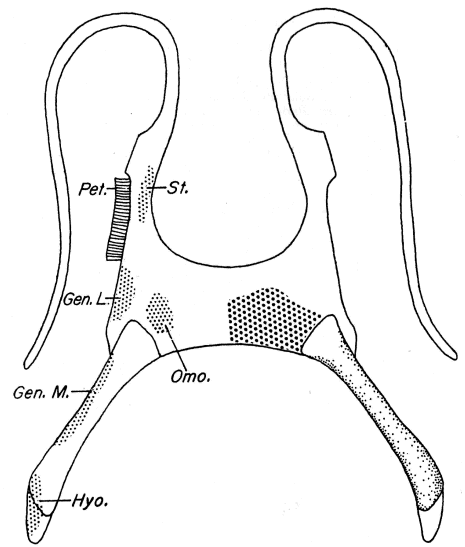 Figure 6 | |
 Figure 8 |  Figure 7 |
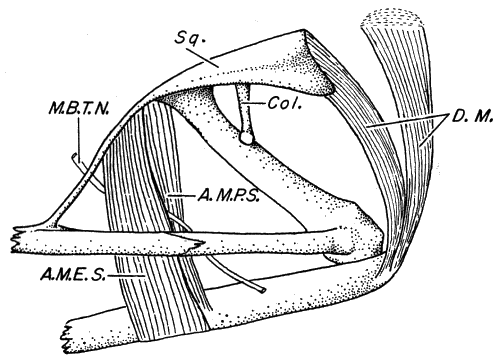 Figure 9 |
Fig. 6. Pipilo erythrophthalmus. Proximal end of left tarsometatarsus and the hypotarsus, × 4.
Fig. 7. Pipilo erythrophthalmus. Lateral view of proximal end of left femur and a portion of the pelvis, × 3.5.
Fig. 8. Pipilo erythrophthalmus. Upper surfaces of the phalanges of the foretoes of the left foot showing insertions of the M. extensor digitorum longus, × 3.
Fig. 9. Pipilo erythrophthalmus. Medial view of the second digit of the left foot, showing insertions of the flexor muscles, × 3.
[Pg 183]
The division of the pars interna of the m. gastrocnemius into
anterior and posterior parts has not been reported by previous
authors yet the division is quite distinct in those birds in which it
occurs. Hudson (1937:36) points out that in some non-passerine
birds the pars interna is double, but that in these species the m.
semimembranosus inserts between the two parts. This is not the
condition in those species studied by me. Only the ploceids and the
cardueline finches in the present investigation fail to show such a
division. The undivided muscle in these birds resembles, in its
origin and position, the posterior portion of the muscle found in
those species showing the bipartite condition. The greater mass
of the bipartite muscle probably makes possible a stronger extension
of the tarsometatarsus.
Thus, the divided or undivided conditions of the m. obturator
externus and the pars interna of the m. gastrocnemius seem to be
correlated with the degrees of strength of certain movements of the
leg. It is conceivable that these differences in structure are correlated
with the manner in which food is obtained, the birds having
the bipartite muscles being those which spend the most time on the
ground searching and scratching for seeds and other sorts of food.
Yet, in Leucosticte, a cardueline, and in Calcarius, an emberizine,
whose foraging habits are rather similar, the structure is unlike.
Leucosticte does resemble the emberizines and also Piranga and
Spzia in the extension of a band of muscle fibers from the pars
interna of the m. gastrocnemius around the front of the knee. A
band of muscle fibers of this sort strengthens the knee joint and
gives still more strength to the pars interna. This condition has
been reported in a number of birds by Hudson (1937) and is, in all
probability, an adaptation for greater strength of certain leg movements.
The development of this band in Leucosticte seems to
parallel that in the other birds studied and does not indicate relationship,
since in Leucosticte this band arises from the undivided
muscle which (as stated above) resembles only the posterior portion
of the bipartite muscle described for the other birds. In the latter,
the muscular band arises from the anterior part of the muscle.
Minor differences in muscle pattern, like those already mentioned,
are consistent also between subfamilies, but correlation of these
minor differences with function is difficult. There is the implication,
however, that in all the groups except the carduelines and
ploceids, the emphasis is on greater strength and mobility of the leg.
In the carduelines that were studied the origin of the m. sartorius
[Pg 184]
does not extend so far craniad as in the other species. In the latter,
at least half of the origin is from the last one or two free dorsal
vertebrae; in the carduelines no more than one third of the origin is
anterior to the ilium. It is conceivable that the more craniad the
origin, the stronger the forward movement of the thigh would be.
In Passer, Estrilda and Poephila, and in all the cardueline finches
examined, the bellies of the m. flexor perforans et perforatus digiti
II and the m. flexor perforans et perforatus digiti III are more intimately
connected than they are in the other species studied. Thus,
the amount of independent action of these muscles in Passer, in
the estrildines, and in the carduelines probably is reduced.
In Passer, the estrildines, and the carduelines the edges of the
sheathlike tendon of insertion of the m. perforatus digiti III are
thickened; as a result the insertion appears superficially to be double
but closer examination reveals that there is a fascia stretched between
the thickened edges. In the other species examined, the
insertion is sheathlike throughout and there are no thick areas. I
cannot explain this on the basis of function. The difference, however,
is obvious and constant.
Aside from the differences noted above, there were variations of
muscle pattern that seem to be significant only in Vireo olivaceus.
In this species the central, aponeurotic portion of the m. iliotibialis
is absent. The origin of the m. adductor longus et brevis is from
the dorsal edge of the ischiopubic fenestra and not from the membrane
covering this fenestra. The origin of the pars posticus of this
muscle, furthermore, is fleshy and not tendinous as it is in the other
species. The m. flexor perforatus digiti II is larger and more deeply
situated in Vireo and has, furthermore, no connection with the m.
flexor hallucis longus. The latter muscle is smaller and weaker than
in any of the other species and has only one (the posterior) head
of origin. The m. flexor hallucis brevis, on the contrary, is larger
than in the other birds, compensating, probably, for the small m.
flexor hallucis longus. In those differences, however, which separate
the carduelines and ploceids from the other birds studied, Vireo
resembles, in every instance, the richmondenines, emberizines, tanagers,
warblers, and blackbirds.
On the basis of differences in leg-musculature the species which
are now included in the Family Fringillidae may be separated into
two groups. One group includes the richmondenines and the emberizines;
the other, the carduelines. The muscle patterns of the
legs of the birds of the first group are indistinguishable from those
of Seiurus, Icterus, Molothrus, and Piranga, and except for the differences
[Pg 185]
noted are similar to those in Vireo. The carduelines, on
the other hand, are similar in every point of leg-musculature to the
ploceids which were studied. Thus, the heterogeneity of the Family
Fringillidae, as now recognized, is emphasized by differences in the
muscle patterns of the leg.
The application of serological techniques to the problems of
animal relationships has been attempted with varying degrees of
success over a period of approximately fifty years. Few of the
earlier studies were of a quantitative nature, but within the past
decade, satisfactory quantitative serological techniques have been
developed whereby taxonomic relationships may be estimated. The
usefulness of comparative serology in taxonomy has been demonstrated
in investigations of many groups wherein results obtained
have, in most instances, been compatible with the results obtained
by more conventional methods, such as comparative morphology.
As Boyden (1942:141) stated, "comparative serology ... is no
simple guide to animal relationship." However, the objectiveness
of its methods, the fact that it has its basis in the comparisons of
biochemical systems which seem to be relatively slow to change in
response to external environmental influences, and the fact that the
results are of quantitative nature favor, where possible, the inclusion
of data from comparative serology along with that from more
conventional sources when an attempt is made to determine the
relationships of groups of animals.
The application of serological methods in ornithology has not
been extensive. Irwin and Cole (1936) and Cumley and Irwin
(1941, 1944) used two species of doves and their hybrids and
demonstrated that a distinction between the red cells of these birds
could be made by use of immunological methods involving the agglutinin
reaction. McGibbon (1945) was able to distinguish the
red cells of interspecific hybrids in ducks by similar methods. Irwin
(1953) used similar techniques in his study of the evolutionary
patterns of some antigenic substances of the blood cells of birds of
the Family Columbidae. Sasaki (1928) demonstrated the usefulness
of the precipitin technique in distinguishing species of ducks
and their hybrids. This technique was used successfully also by
DeFalco (1942) and by Martin and Leone (1952). Working with
groups of known relationships, these investigators showed that the
"accepted" systematic positions of certain birds were confirmed by
[Pg 186]
serological procedures. The precipitin reaction, however, has never
been applied to actual problems in avian taxonomy prior to the
present study.
Although most previous work in comparative serology in which precipitin
tests were used has involved the use of whole sera as antigens, Martin and
Leone (1952) indicated that tissue extracts are satisfactory as antigens and
that serological differentiation can be obtained with these extracts and the
antisera to them. I decided, therefore, to use such extracts in these investigations,
since the small sizes of the birds to be tested made it impracticable to
obtain enough whole sera.
Most of the birds used were obtained by shooting, but a few were trapped
and the exotic species were purchased alive from a pet dealer. When a bird
was killed, the entire digestive tract was carefully removed to prevent the
escape of digestive enzymes into the tissues and to prevent putrefaction by
action of intestinal bacteria. As soon as possible (and within three hours in
every instance) the bird was skinned, the head, wings, and legs were removed,
and the body was frozen. Each specimen, consisting of trunk, heart, lungs,
and kidneys, was wrapped separately and carefully in aluminum foil to prevent
dehydration of the tissues. The specimens were kept frozen until the time
when the extracts were made.
When an extract was to be prepared, the specimen was allowed to thaw but
not to become warm. In the cold room with the temperature of all equipment
and reagents at 2°C., the specimen was placed in a Waring blender with 0.9
per cent aqueous solution of NaCl buffered with M/150 K2HPO4 and M/150
Na2HPO4 to a pH of 7.0. The amount of reagent used was 75 ml. of saline for
each gram of tissue to be extracted. The tissues were minced in the blender,
allowed to stand at 2°C. for 72 hours, and the tissue residues removed by
centrifugation in a refrigerated centrifuge. Formalin was added to a portion of
the supernatant in the amount necessary to make the final dilution 0.4 per cent.
This formolization was found to be necessary to inhibit the action of autolytic
enzymes over the period of time required to complete the investigations. The
effects of formolization on the antigenicity and reactivity of proteins are discussed
later. It was necessary to sterilize and clarify the "native" (unformolized)
extracts; this was done by filtration through a Seitz filter. These "native"
substances were used only in the early stages of the investigation (see below).
The filtrate was bottled and stored at 2°C. In the early stages of this investigation
clarification of the formolized extract was accomplished by the same
sort of filtration. It was determined, however, that centrifugation in a refrigerated
centrifuge at high speeds (17,000g) served the same purpose and
was quicker. The formolized extracts were bottled and also stored at 2°C.
(although refrigerated storage of the formolized extracts does not seem necessary).
For each extract the amount of protein present was determined colorimetrically
by the method of Greenberg (1929) with a Leitz Photrometer.
Species for which extracts were prepared and the protein values of the
extracts are listed in Table 1. Extracts of some species were used throughout
most of the experiment; extracts of others were used only when needed for
purposes of comparison.
[Pg 187]
Table 1.—Species from Which Extracts Were Prepared and Injection
Schedules for Extracts Against Which Antisera Were Produced
| Species | Protein, gms. per 100 ml | Injection schedules for production of antisera |
| Myiarchus crinitus (Linnaeus) | 0.65 | Series 1: Intravenous, 0.5, 1.0, 2.0, and 4.0 ml. |
| Passer domesticus | 1.40 | Series 1: Subcutaneous, 0.5, 1.0, 2.0, and 4.0 ml. |
| Estrilda amandava | 0.45 | [A]Series 1: Intravenous, 0.5, 1.0, 2.0, and 4.0 ml. [A]Series 2: Subcutaneous, 0.5, 1.0, and 2.0 ml. Intraperitoneal, 8.0 ml. |
| Poephila guttata | 0.56 | [A]Same as for Estrilda. |
| Molothrus ater | 0.65 | Series 1: Intravenous and subcutaneous, respectively, 0.5 and 0.5 ml., 1.0 and 1.0 ml., 3.0 and 1.0 ml., 5.0 and 3.0 ml. Series 2: Subcutaneous, 0.5, 1.0, 2.0 and 4.0 ml. |
| Piranga rubra | 0.50 | Same as for Molothrus. |
| Richmondena cardinalis | 0.70 | [A]Same as for Estrilda. |
| Richmondena cardinalis | 0.60 | Same as for Spinus. |
| Passerina cyanea | 0.45 | Antiserum not prepared. |
| Spiza americana | 0.70 | Same as for Molothrus. |
| Carpodacus purpureus | 0.50 | Antiserum not prepared. |
| Spinus tristis | 0.49 | Series 1: Intravenous, 0.5, 1.0, 2.0, and 4.0 ml. Series 2: Intravenous, 0.5, 1.0, 2.0, and 4.0 ml. Series 3: Subcutaneous, 0.5, 1.0, 2.0, and 4.0 ml. |
| Pipilo erythrophthalmus | 0.92 | Antiserum not prepared. |
| Junco hyemalis | 0.56 | Same as for Spinus. |
| Spizella arborea | 0.48 | Same as for Spinus. |
| Zonotrichia querula | 0.48 | Same as for Spinus. |
| Zonotrichia albicollis (Gmelin) | 0.92 | Antiserum not prepared. |
[A] Antiserum prepared against formolized antigen.
[Pg 188]
All antisera were produced in rabbits (laboratory stock of Oryctolagus
cuniculus). Three methods of injection of antigen were used in various combinations:
intravenous, subcutaneous, and intraperitoneal. Injection schedules
used in the production of each antiserum are listed in Table 1. Both formolized
and "native" antigens were used. Each rabbit received one or more series
of four injections, each injection being administered on alternate days and doubling
in amount: 0.5 ml., 1.0 ml., 2.0 ml., and 4.0 ml. In all but two instances
more than one series of injections was necessary to produce a useful antiserum.
More than two series, however, resulted in little or no improvement of the
reactivity of the antiserum.
The injection-series were separated by intervals of eight days. On the eighth
day after the last injection of each series, 10 ml. of blood were withdrawn from
the main artery of the ear of the rabbit, and the antiserum was used in a
homologous precipitin test to determine its usefulness. If the antiserum contained
sufficient amounts of antibodies to conduct the projected tests, the rabbit
was completely exsanguinated by cardiac puncture, by using an 18-gauge needle
and a 50 ml. syringe. The whole blood was placed in clean test tubes and
allowed to clot. It was allowed to stand at 2°C. for 12 to 18 hours so that
most of the serum would be expressed from the clot. The serum was then
decanted, centrifuged to remove all blood cells, sterilized in a Seitz filter,
bottled in sterile vials, and stored at 2°C. until used.
The precipitin reaction is the most successful of the serological techniques
thus far devised for systematic comparisons. The reaction occurs because
antigenic substances introduced into the body of an animal cause the formation
of antibodies which precipitate antigens when the two are mixed. The antisera
which are produced show quantitative specificities in their actions; therefore,
when an antiserum containing precipitins is mixed with each of several antigens,
the reaction involving the homologous antigen (that used in the production of
the antiserum) is greater than those reactions involving the heterologous antigens
(antigens other than those used in the production of the antiserum).
Furthermore, the magnitudes of the reactions between the antiserum and the
heterologous antigens vary according to the degrees of similarity of these
antigens to the homologous one.
The method of precipitin testing follows that outlined by Leone (1949). The
Libby (1938) Photronreflectometer was used to measure the turbidities developed
by the interaction of antigen and antiserum. With this instrument
parallel rays of light are passed through the turbid systems being measured.
Light rays are reflected from the suspended particles to the sensitive plate of a
photoelectric cell; this generates a current of electricity which causes a deflection
on a galvanometer. The deflection is proportional to the amount of turbidity
developed and readings may be taken directly from the scale of the instrument.
The reaction-cells of the photronreflectometer are designed to operate with
a volume of 2 ml.; therefore, this volume was used in all testing. In every
series of tests the amount of antiserum was held constant and the amount of
antigen was varied. The volume for each antigen dilution was always 1.7 ml.,
and to this was added 0.3 ml. of antiserum to make up a volume of 2 ml.
[Pg 189]
Table 2.—Percentage values obtained from analyses of precipitin reactions.
Numerals represent relative amounts of reaction between antigens and antisera.
Homologous reactions are arbitrarily valued as 100 per cent, and heterologous
reactions are expressed accordingly. Comparisons are meaningful only if made
within each horizontal row of values.
| Antigens | ANTISERA | |||||||
| | ||||||||
| Passer domesticus | 75 | 74 | 73 | 66 | 81 | 72 | ... | 81 |
| Estrilda amandava | 100 | 88 | 75 | ... | 79 | 72 | 53 | ... |
| Poephila guttata | 95 | 100 | 77 | 67 | 87 | 81 | ... | ... |
| Molothrus ater | 66 | 54 | 69 | 65 | 86 | 75 | 69 | 75 |
| Piranga rubra | ... | ... | 100 | ... | ... | ... | ... | 89 |
| Richmondena cardinalis | 75 | 80 | 91 | 100 | 98 | 65 | 88 | 91 |
| Spiza americana | 65 | 68 | ... | 71 | 100 | 64 | 67 | 80 |
| Carpodacus purpureus | 70 | 71 | 71 | 61 | 89 | 93 | 53 | 70 |
| Spinus tristis | 72 | 74 | 73 | 60 | 89 | 100 | 60 | ... |
| Junco hyemalis | 64 | 56 | 74 | 65 | 87 | 68 | 100 | ... |
| Zonotrichia querula | 65 | 71 | ... | 67 | 89 | 75 | ... | 100 |
Antigens were diluted with 0.9 per cent phosphate-buffered saline solution.
Tests were run in standard Kolmer test-tube racks, each test consisting of 12
tubes. Each dilution was made on the basis of the known protein concentration
of the antigen. The first tube contained an initial dilution of 1 part protein
in 250 parts saline and each successive tube contained a protein dilution one-half
the concentration of the preceding tube, ranging up to 1:512,000. Saline
controls, antiserum controls, and antigen controls were maintained with each
test to determine the turbidities inherent in these solutions. These control-turbidities
were deducted from the total turbidity developed in each reaction-tube,
the resultant turbidity then being considered as that which was caused
by the interaction of antigens and antibodies. The turbidities were allowed to
develop over a 24-hour period. In the early stages of this investigation the
reactions were allowed to take place at 2°C. in order to inhibit bacterial growth.
[Pg 190]
Later tests were carried out at room temperatures, and bacterial growth was
prevented by the addition to each tube of 'Merthiolate' in a final dilution of
1:10,000.
Corrected values for the turbidities obtained were plotted with the turbidity
values on the ordinate and the antigen dilutions on the abscissa. The homologous
reaction was the standard of reference for all other test reactions with the
same antiserum. By summing the plotted turbidity readings, numerical values
are obtained which are indices serving to characterize the curves. Such values
were converted to percentage values, that of the homologous reaction being
considered 100 per cent. These values, plus the curves, provide the data by
means of which the proteins of the birds may be compared. Plots representative
of the precipitin curves are presented in Figs. 10 to 21. For convenience
each plot represents only several of the 10 curves obtained with each antiserum.
A summary of the serological relationships of the birds involved in the
precipitin tests is presented in Table 2, in which percentage values are presented.
Since the techniques involved in testing were greatly improved as the
investigation proceeded, the summary is based solely on those tests run in the
later stages of the investigation. For reasons which will become apparent in
later discussion, it should be emphasized that in Table 2 comparisons may be
made only within each horizontal row of values.
One of the problems met early in this investigation was instability
of the proteins in the extracts that were prepared. Extracts in which
no attempt was made to inactivate the enzymes present proved unsatisfactory.
It was necessary to maintain the temperature of the
"native" antigens at 2°C, and all work with such antigens had to be
performed at this temperature. This arrangement was inconvenient;
furthermore, inactivation of the enzymes was not complete even at
this low temperature, and some denaturation of the proteins took
place as evidenced by the gradual appearance of insoluble precipitates
in the stored vials.
The preservatives, 'Merthiolate' and formalin, were used in an
attempt to inhibit the autolytic action of the enzymes present.
Formalin, when added to make a final dilution of 0.4 per cent,
proved to be the more satisfactory of the two preservatives and was
used throughout most of the work. Formalin caused slight denaturation
of some of the proteins, but this effect was complete
within a few hours, after which any denatured material was removed
by filtration or centrifugation. The proteins remaining in
solution were stable over the period necessary to complete the investigations.
The addition of formalin reduces the reactivity of the extracts
when they are tested with antisera prepared against "native" antigens
[Pg 191]
and causes changes in the nature of the precipitin curves. This
effect has been pointed out by Horsfall (1934) and by Leone (1953)
in their work on the effects of formaldehyde on serum proteins.
Their data indicate, however, that even though changes in the
immunological characteristics of proteins are brought about by formolization,
the proteins retain enough of their specific chemical characteristics
to allow consistent differentiation of species by immunological
methods. In the tests which I performed, the relative positions
of the precipitin curves, whether native or formolized extracts
were involved, remained unchanged (Figs. 10, 11). All data used
in interpretation of the serological relationships were obtained from
tests in which formolized antigens of equivalent age were used.
Only three antisera were produced against formolized antigens,
all others being produced against "native" extracts. The formolized
antigens seemed to have a greater antigenicity, in most instances,
than did those which were unformolized, and precipitin reactions
involving antisera produced against formolized antigens developed
higher turbidities. The antisera produced against formolized antigens
were equal to but no better than those prepared against "native"
extracts in separating the birds tested (Figs. 12, 13).
The rabbit is a variable to be considered in serological tests. Two
rabbits exposed to the same antigen, under the same conditions, may
produce antisera which differ greatly in their capacities to distinguish
different antigens. It is logical to assume, therefore, that
two rabbits exposed to different antigens may produce antisera
which also differ in this respect. This explains the unequal values
of reciprocal tests shown in Table 2. Thus, in the test involving
the antiserum to the extracts of Richmondena, a value of 71 per cent
was obtained for Spiza antigen, whereas in the test involving anti-Spiza
serum, a value of 98 per cent was obtained for Richmondena
antigen. In Table 2, therefore, comparisons may be made only
among values for the proteins of birds tested with the same antiserum.
Since the amount of any one antiserum is limited, there is, of necessity,
a limit as to the number of birds used in a series of serological
tests. Therefore, although the results reveal the actual serological
relationships of the individual species, interpretation of the relationships
of the taxonomic groups must be undertaken with the realization
that such an interpretation is based on tests involving relatively
few species of each group. It is reasonable to assume, however,
that a species which has been placed in a group on the basis of resemblances
other than serological resemblance would show greater
[Pg 192]
serological correspondence to other members of that group than it
would to members of other groups. Specifically, in the Fringillidae
and their allies, there seems to be little reason to doubt that genera,
and even subfamilies, are natural groups. This is illustrated in tests
involving closely related genera: Richmondena and Spiza (Figs.
14, 15, 18), Estrilda and Poephila (Fig. 21), Spinus and Carpodacus
(Figs. 12, 17, 19, 20). In each of these tests the pairs of genera
mentioned show greater serological correspondence to each other
than they do to other kinds involved. This point is illustrated further
by a test (not illustrated) involving Zonotrichia querula (the
homologous antigen) and Zonotrichia albicollis. Although this test
was one of an earlier series in which difficulties were encountered
(the data, therefore, were not used), it is of interest that the two
species were almost indistinguishable serologically.
The serological homogeneity of passeriform birds is emphasized
by the fact that the value of every heterologous reaction was more
than 50 per cent of the value of the homologous reaction, except in
the test involving the anti-Richmondena serum and Myiarchus (Fig.
13) in which the value of the heterologous reaction was 45 per cent.
Because most ornithologists consider these genera to be only distantly
related (they are in different suborders within the Order
Passeriformes), the relatively high value of the heterologous reaction
emphasizes the close serological correspondence of passerine
birds and indicates that small consistent serological differences
among these birds are actually significant. The possibility that
some of the serological correspondence is due to the "homologizing"
effect of formalin on proteins should not be excluded. I think, however,
that this effect is not entirely responsible for the close correspondence
observed here.
An additional point to consider in interpretation of the serological
tests is that the techniques used tend to separate sharply species that
are closely related whereas species that are distantly related are not
so easily separated. In other words, comparative serological studies
with the photronreflectometer tend to minimize the differences between
distant relatives and to exaggerate the differences between
close relatives.
In analyzing the serological relationships of the species used in
this study, it becomes obvious that two or more series of tests must
be considered before the birds can be placed in relation to each
other. For example, the data presented in Fig. 14 indicate that
Spiza and Molothrus show approximately the same degree of serological
correspondence to Richmondena. This does not imply necessarily
[Pg 193]
that Spiza and Molothrus are closely related. If Fig. 15 is
examined, it can be determined that Richmondena shows much
greater serological correspondence to Spiza than does Molothrus.
Thus, an analysis of both figures serves to clarify the true serological
relationships of the three genera. By reference to other series of
tests involving these three birds a more exact determination of their
relationships may be obtained.
To illustrate this point by a hypothetical example, two species
might seem equidistant, serologically, from a third species. Additional
testing should indicate if the first two species are equidistant
in the same direction (therefore, by implication, close relatives) or
in opposite directions (therefore, distant relatives). A single test
supplies only two dimensions of a three dimensional arrangement.
It is impossible to interpret and to picture the serological data
satisfactorily in two dimensions; therefore, a three-dimensional
model (Figs. 22, 23) was constructed to summarize the serological
relationships of the birds involved. Each of the eleven kinds used
consistently throughout the investigation is represented in the model.
By use of the percentage values (Table 2), each bird was located
in relation to the other birds. Where possible, averages of reciprocal
tests (Table 3) were used in determining distances between the
elements of the model. In this way seven of the birds were accurately
located in relation to each other. Lacking reciprocal tests,
the positions of the other birds were determined by the values of
single tests (Table 4). Although these birds were placed with less
certainty, at least four points of reference were used in locating each
species. At least one serological test is represented by each connecting
bar in the model. The lengths of the bars connecting any
two elements were determined as follows: a percentage value
(Table 3 and Table 4) representing the degree of serological correspondence
between two birds was subtracted from 100 per cent;
the remainder was multiplied by a factor of five to increase the size
of the model and the product was expressed in millimeters; a bar
of proper length connects the two elements involved.
From the model it is observed that, Molothrus and Passer excluded,
the birds fall into two distinct groups: one includes Piranga,
Richmondena, Spiza, Junco, and Zonotrichia; the other includes
Estrilda, Poephila, Carpodacus, and Spinus.
[Pg 194]
Table 3.—Reciprocal Values Used to Determine Distances Between
Elements of the Model; Each Value Represents the Average of Serological
Tests Between the Species Involved
| | ||||||||
| Estrilda amandava | .. | 92 | .. | 72 | 72 | 59 | .. | |
| Poephila guttata | 92 | .. | 74 | 78 | 78 | .. | .. | |
| Richmondena cardinalis | .. | 74 | .. | 85 | 63 | 77 | 79 | |
| Spiza americana | 72 | 78 | 85 | .. | 77 | 77 | 85 | |
| Spinus tristis | 72 | 78 | 63 | 77 | .. | .. | .. | |
| Junco hyemalis | .. | .. | 77 | 77 | .. | .. | .. | |
| Zonotrichia querula | .. | .. | 79 | 85 | .. | .. | .. | |
of the Model; Each Value Represents a Single Test Between the
Species Involved
| Passer domesticus | .. | 74 | 73 | .. | 72 | .. | .. | |
| Molothrus ater | .. | 54 | .. | 65 | .. | 69 | 75 | |
| Piranga rubra | .. | 77 | .. | 91 | 73 | 74 | .. | |
| Carpodacus purpureus | 70 | 71 | .. | 61 | 93 | .. | .. | |
[Pg 195]
 |  |
 | 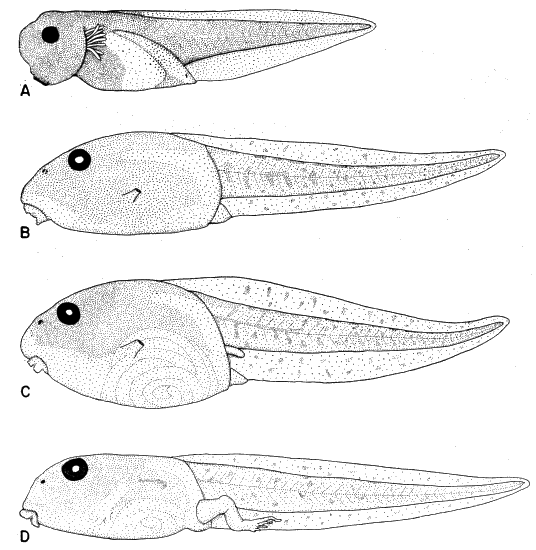 |
Figs. 10-13. Graphs of precipitin reactions illustrating effects of formalin on antigenicity and reactivity of the extracts. For further information, see text, pp. 190-193.
Fig. 10. Reactions of unformolized antigens of Richmondena, Zonotrichia, and Molothrus with anti-Richmondena serum. Fig. 11. Reactions of formolized antigens of Richmondena, Zonotrichia, and Molothrus with anti-Richmondena serum. Fig. 12. Reactions of anti-Richmondena serum prepared against native antigen with antigens of Richmondena, Zonotrichia, Carpodacus, and Spinus. Fig. 13. Reactions of anti-Richmondena serum prepared against formolized antigen with antigens of Richmondena, Zonotrichia, Poephila, Spinus, and Myiarchus.
[Pg 196]
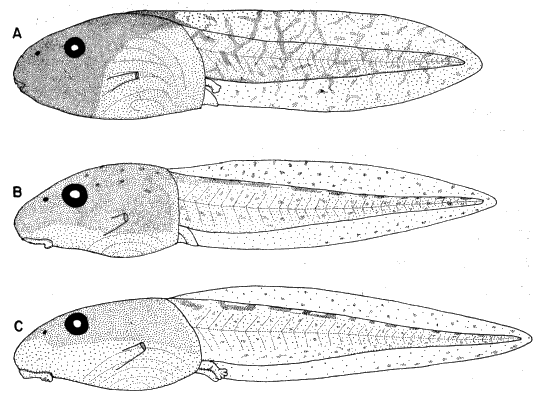 |  |
 | 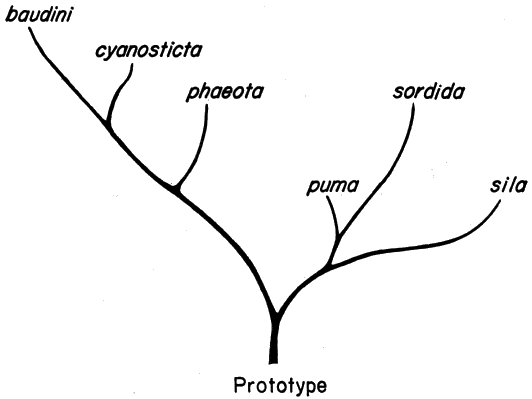 |
Figs. 14-17. Graphs of precipitin reactions illustrating serological relationships. For further explanation, see text, pp. 190-193.
Fig. 14. Serological relationships of Richmondena, Spiza, and Molothrus. Fig. 15. Serological relationships of Richmondena, Spiza, and Molothrus. Fig. 16. Serological relationships of Carpodacus with the richmondenine-emberizine-thraupid assemblage. Fig. 17. Serological relationships of Carpodacus and Spinus with Richmondena and Junco.
[Pg 197]
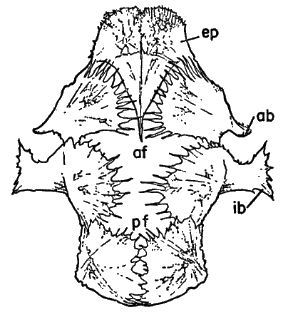 | 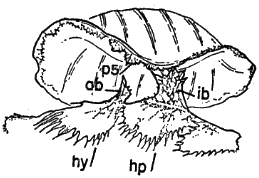 |
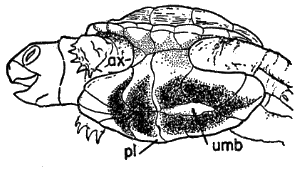 | 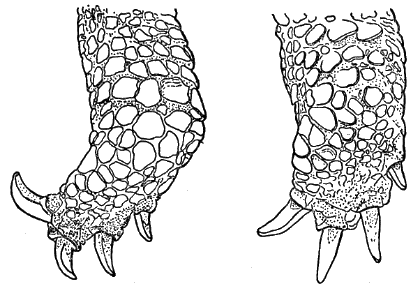 |
Figs. 18-21. Graphs of precipitin reactions illustrating serological relationships. For further explanation, see text, pp. 190-193.
Fig. 18. Serological relationships of Spinus and Poephila with the richmondenines. Fig. 19. Serological relationships of Carpodacus and Spinus with Richmondena and Piranga. Fig. 20. Serological relationships of Poephila and Richmondena with the carduelines. Fig. 21. Serological relationships of Richmondena and Spinus with the estrildines.
[Pg 198]
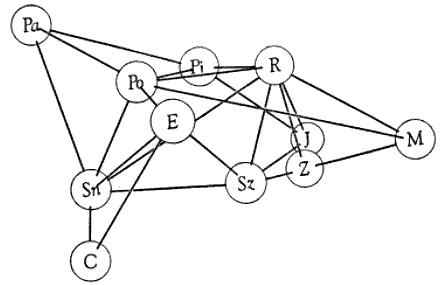
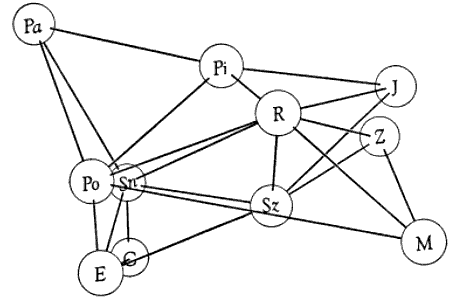
Fig. 22. Two views of a model illustrating serological relationships among fringillid and related birds. For further explanation, see text, pp. 193-194.
| Genera | Pi | . . . . | Piranga | |||
| C | . . . . | Carpodacus | Po | . . . . | Poephila | |
| E | . . . . | Estrilda | R | . . . . | Richmondena | |
| J | . . . . | Junco | Sn | . . . . | Spinus | |
| M | . . . . | Molothrus | Sz | . . . . | Spiza | |
| Pa | . . . . | Passer | Z | . . . . | Zonotrichia | |
[Pg 199]
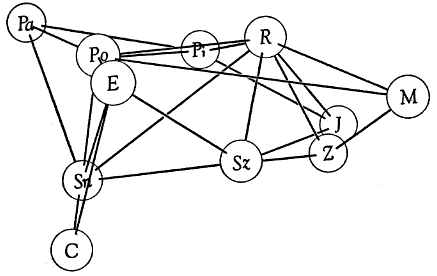
Fig. 23. Two additional views of the model shown in fig. 22 illustrating serological relationships among fringillid and related birds. For further explanation, see text, pp. 193-194.
| Genera | Pi | . . . . | Piranga | |||
| C | . . . . | Carpodacus | Po | . . . . | Poephila | |
| E | . . . . | Estrilda | R | . . . . | Richmondena | |
| J | . . . . | Junco | Sn | . . . . | Spinus | |
| M | . . . . | Molothrus | Sz | . . . . | Spiza | |
| Pa | . . . . | Passer | Z | . . . . | Zonotrichia | |
[Pg 200]
Within the richmondenine-emberizine-thraupid assemblage, Junco
and Zonotrichia constitute a sub-group apart from the others.
Piranga and Richmondena show close serological correspondence.
The present taxonomic position of Spiza in the Richmondeninae,
which has been questioned by Beecher (1951a:431; 1953:309), is
corroborated at least insofar as the serological evidence is concerned.
Certainly, serological correspondence of Spiza with the richmondenine-emberizine-thraupid
assemblage is greater than with any
other group of birds tested.
It is obvious that the serological affinities of the carduelines do
not lie with the richmondenines, emberizines, or thraupids. The
carduelines show greater serological correspondence with the
estrildines than they do with any of the other groups tested. Further
serological investigation involving other species, however, is
necessary before the nearest relatives of the carduelines can be determined
with certainty.
The two estrildines tested (Estrilda and Poephila) show close
serological relationship. Their nearest relatives, serologically, seem
to be the carduelines. The classification (Wetmore, 1951) that
places Passer in the same family with the estrildines is not upheld by
the serological data available. Passer is not, serologically, closely
related to any of the birds tested. It is of interest that Beecher
(1953:303-305), on the basis of jaw musculature, places Passer and
the estrildines in separate families (Ploceidae and Estrildidae, respectively).
Molothrus shows greater serological correspondence to the richmondenine-emberizine-thraupid
assemblage than to any of the other
birds tested. It is definitely set apart from this group, however, and
its position, serologically, is compatible with that based on evidence
from other sources.
There seems to be but little argument among ornithologists that
icterids, fringillids, and ploceids constitute families which are distinct
from one another. If, then, the serological differences between
Molothrus (Icteridae) and Richmondena (Fringillidae), between
Molothrus and Zonotrichia (Fringillidae), and between Richmondena
and Poephila (Ploceidae) are indicative of family differences,
there are four families represented by the birds involved.
Molothrus represents one family; Piranga, Richmondena, Spiza,
Junco, and Zonotrichia, a second; Estrilda, Poephila, Carpodacus,
and Spinus, a third; and Passer, a fourth.
[Pg 201]
The heterogeneity of the Family Fringillidae has been emphasized
by many authors. The relationships of the species now included
in this Family have been the subject of much discussion and constitute
an important problem in avian systematics.
Sushkin's studies (1924, 1925) of features of the horny and bony
palates have served as a basis for the present division of the Family
into subfamilies. Recently, Beecher (1951a, 1951b, 1953) and
Tordoff (1954) have used these features and others which they
thought to be of value in an attempt to clarify the relationships of
the species involved.
Beecher's work (1951a, 1951b, 1953) on jaw-musculature is a
valuable contribution to our knowledge of the anatomy of passerine
birds. His myological studies were so thorough and his presentation
so detailed that students who disagree with his interpretations
can draw their own conclusions. Beecher (1951b:276) points out
that there are two basic types of skeletal muscle—those with parallel
fibers and those with pinnately arranged fibers. The muscles with
pinnate fibers seem to be more efficient, each muscle having a
greater functional cross section for its bulk than does one with
parallel fibers. He assumes that muscles with parallel fibers are
more primitive, phylogenetically, than are those with fibers arranged
pinnately. Since his study of the jaw muscles of the Icteridae
(1951a) revealed that patterns of jaw-musculature within this
Family remain constant regardless of the methods used in procuring
food, he assumes that such patterns may be used as indicators
of relationship throughout the entire oscinine group. These two
assumptions, then, serve as the basis for his hypothesis concerning
relationship and phylogeny within this assemblage. Beecher
(1951b:278-280; 1953:310-312) maintains that within the Family
Thraupidae there are two main lines which lead with almost no disjunction
to the Carduelinae and Richmondeninae. The thraupid-richmondenine
line involves a shift in the nature of the m. adductor
mandibulae externus superficialis, which becomes more pinnate in
the richmondenines. This results in greater crushing power. The
thraupid-cardueline line involves a shift in emphasis from the the m.
adductor mandibulae externus medialis to the m. pseudotemporalis
superficialis and the forward advance of the insertion of the latter.
This, also, promotes greater crushing ability. He states that features
of the horny palate and of the plumage provide further evidence of
close relationship of these groups. He includes, therefore, the
[Pg 202]
Thraupinae, the Carduelinae, and the Pyrrhuloxiinae (=Richmondeninae)
in the Family Thraupidae. Beecher (1953:307) indicates
that the patterns of jaw-musculature of the Parulinae (wood warblers)
and Emberizinae (buntings) are similar and suggests that the
buntings had their origin from the wood warblers. He includes
these subfamilies, therefore, in the Family Parulidae.
Beecher's reasoning may be criticized on several points. It may
be, as he suggests, that muscles with parallel fibers evolved earlier,
phylogenetically, than did muscles with pinnate fibers, but he does
not give adequate consideration, it seems to me, to the possibility
that parallel fibers may also have evolved secondarily from pinnate
fibers. Since Beecher (1951a) found that patterns of jaw-musculature
within the Family Icteridae were conservative, he is reluctant
to admit the possibility of convergence among any of the other
families. Differences in patterns of jaw-musculature are, however,
functional adaptations and like the bill, which is also associated with
food-getting may be subject to rapid evolutionary change. Finally,
in attempting to classify the oscines, he has relied almost entirely on
a single character—the pattern of jaw-musculature.
Tordoff's attempts (1954) to clarify the relationships of the fringillids
and related species are based chiefly on features of the bony
palate. He assumes that since palato-maxillaries seem to be absent
in the majority of passerine birds, their occurrence in certain nine-primaried
oscine groups indicates relationship among these groups.
He points out that these bones, when present, are important areas
of origin of the m. pterygoideus which functions in depression of
the upper jaw and in elevation of the lower jaw. He assumes, therefore,
that palato-maxillaries were evolved to provide for a more
effective action of the m. pterygoideus. The need for such action
could be associated with a seed-eating habit. All richmondenines
and emberizines possess palato-maxillary bones either free or fused
to the prepalatine bar, but there is no trace of these bones in the
carduelines. Carduelines, furthermore, possess prepalatine bars
that are characteristically flared anteriorly. This condition does not
exist in the richmondenines or in the emberizines.
Tordoff points out, also, that the irregular, erratic migrations of
the New World Carduelinae are unlike the more regular migrations
of the richmondenines and emberizines. The carduelines, furthermore,
are more arboreal in their habits than are these other groups
and exhibit a decided lack of nest sanitation during the later stages
of nesting, a situation which contrasts with that found in the Richmondeninae
[Pg 203]
and Emberizinae. He suggests, therefore, that the
carduelines are not so closely related to the richmondenines and the
emberizines as previously has been thought.
Since there are only two cardueline genera, Loximitris and Hesperiphona,
endemic to the New World and at least 10 genera with
many species endemic to the Old World, Tordoff (1954:15) suggests
an Old World origin for the carduelines. He strengthens his
argument for this hypothesis by pointing out that in features of the
bony palate and in habits the carduelines resemble the estrildines of
the Family Ploceidae.
Tordoff (1954:29-30) states that the tanagers not only merge with
the richmondenines but also grade imperceptibly into the emberizines.
He includes, therefore, the Richmondeninae, Emberizinae,
and Thraupinae in the Family Fringillidae. He suggests that the
carduelines are ploceids, closely related to the Subfamily Estrildinae,
on the basis of structure of the bony palate, geographic distribution,
social behavior, and habits such as nest-fouling and nest-building.
Tordoff, like Beecher, has based his interpretations chiefly on one
feature—structure of the bony palate. Since this feature also is
associated with food-getting, the possibilities of convergence of distantly
related species with similar habits and divergence of closely
related species with different habits may not be excluded.
The hazard of unrecognized adaptive convergence cannot, of
course, be excluded from most fields of taxonomic research, but
some features of morphology and biochemistry are notably more
conservative than others and undergo slower evolutionary change.
Such features are often of utmost importance in distinguishing the
higher taxonomic categories.
Most ornithologists are aware that, within the Order Passeriformes,
patterns of musculature in the leg have evolved at a slow
rate and exhibit little variation within the Order. Differences which
do occur, therefore, probably are significant, especially those that
are consistent between groups of species. As I have pointed out
earlier (p. 184), there are no significant differences in leg-musculature
between the Richmondeninae, Emberizinae, and Thraupidae. Indeed,
it is difficult to define these groups on the basis of leg-musculature.
If these groups are of common origin, the lack of distinct
boundaries between them is not surprising. A muscular band which
extends from the pars interna of the m. gastrocnemius around the
front of the knee is present in every emberizine species that I studied
and in the Genus Piranga. With the exception of Spiza none of the
richmondenines possesses this band.
[Pg 204]
The significant differences in leg-musculature which have been
discussed above (pp. 183-184) distinguish the carduelines from the
New World finches and tanagers. Even the cardueline Leucosticte
and the emberizine Calcarius, which resemble one another in general
adaptations and in several myological features of the leg (p.
183), agree in significant features of the musculature with the respective
groups to which they belong. The carduelines agree in
the major features of leg-musculature with the ploceids which I
studied.
The use of serological techniques in taxonomic work has two
main advantages. The biochemical systems involved in such investigations
seem to be relatively slow to change in response to external
environmental influences, and the quantitative nature of the
results obtained makes possible objective measurement of resemblances
among species.
I have pointed out (p. 200) that the carduelines are excluded,
serologically, from the distinct assemblage formed by the richmondenines,
emberizines, and tanagers. Actually, the carduelines show
less serological resemblance to this assemblage than do the estrildines,
and most ornithologists agree that the Estrildinae are not at
all closely related to the Richmondeninae, Emberizinae, and Thraupidae.
Molothrus, representing a family (Icteridae) recognized as
distinct from the Family Fringillidae, also more closely resembles
the fringillid assemblage, serologically, than do the carduelines. Although
the Carduelinae constitute a distinct group serologically,
they show greater serological resemblance to the estrildines of the
Family Ploceidae than to any of the other species tested. At least
the carduelines and the estrildines form a group as compact as the
subfamilies of the Fringillidae. Thus, the serological data correlate
well with those obtained from the study of the leg-musculature.
Present systems of classification include the subfamilies Passerinae
and Estrildinae in the Family Ploceidae. Passer, however, is less
closely related to the estrildines serologically than are the carduelines,
and is less closely related to the estrildines than Molothrus, an
icterid, is to the fringillids. This raises a question as to the homogeneity
of the Family Ploceidae as presently recognized by most
ornithologists. If the Passerinae and the Estrildinae are placed in
a single family, the serological divergence among members of this
group is certainly greater than it is in the Family Fringillidae. Additionally,
Beecher (1953:303-304) found that the estrildines possess
a pattern of jaw-musculature different from those in other ploceids.
[Pg 205]
The combined evidence from jaw-musculature and serology has
caused me to conclude that the estrildines should be excluded from
the Family Ploceidae (see below).
In an attempt to clarify the relationships of the Fringillidae and
allied groups, I here review briefly the evidence which has been
presented. From his studies of jaw-musculature (1951a, 1951b,
1953) Beecher concludes that the Pyrrhuloxinae (=Richmondeninae),
the Carduelinae, and the Thraupinae are closely related.
He places these groups in the Family Thraupidae. He excludes the
Emberizinae from this group and places them with the wood warblers
in the Family Parulidae. He suggests that the estrildines constitute
a family (Estrildidae) separate from the Family Ploceidae.
From his studies of certain features of the bony palate Tordoff
(1954:25-26, 32) concludes that the richmondenines, the emberizines,
and the tanagers have a common origin and places these
groups in the Family Fringillidae. He excludes the carduelines from
this assemblage, suggests that they are closely related to the estrildines,
and includes them as the Subfamily Carduelinae in the
Family Ploceidae.
In this paper I have presented data obtained from the study of
certain features of morphology and biochemistry which I think are
less subject to the influence of environmental factors than those
features studied by recent workers. It is significant that the data
obtained by use of serological techniques and those obtained from
the study of leg-musculature point to the same conclusions. On the
basis of these data I have drawn several conclusions concerning the
relationships of the groups which I studied.
The richmondenines, emberizines, and tanagers are closely related
and should be included in a single family, Fringillidae. The
Carduelinae and the Estrildinae are closely related subfamilies. Although
most recent classifications place the Estrildinae and Passerinae
in the Family Ploceidae, the serological evidence indicates
that these groups are not closely related. Beecher (1953:303-304)
drew the same conclusion from his study of jaw-musculature (see
above). I suggest, therefore, that the Carduelinae and the Estrildinae
be placed in a family separate from the Ploceidae and that
the name Carduelidae (rather than Estrildidae) be used for this
group. At present, neither is an accepted family name. Because
Carduelis Brisson 1760 is an older name than Estrilda Swainson
1827 and because Carduelis seems to be a centrally located genus
in the family, I have chosen the former (although the International
[Pg 206]
Rules of Zoological Nomenclature do not specify that priority must
apply in forming family names).
I have been unable to study any of the species included in the
subfamilies Fringillinae (not Fringillinae of Tordoff, see 1954:23-24,
and below) and Geospizinae of recent classifications; thus these
groups have not been discussed above. Beecher (1953:307-308)
includes Fringilla in the Subfamily Carduelinae; he includes the
geospizines in a separate family, Geospizidae, and states that they
are derived from the emberizines. Tordoff (1954:23-24) found that
in features of the bony palate Fringilla and the geospizines resemble
the emberizines and, on this basis, includes them in the Subfamily
Fringillinae.
The Dickcissel, Spiza americana, possesses certain features which
merit special discussion. Beecher (1951a:431; 1953:309), on the
basis of jaw-musculature, considers it an icterid. To be sure Spiza is
in many ways an aberrant member of the group to which it is now
assigned (Subfamily Richmondeninae). Spiza, serologically, is
closely related to all species of the richmondenine-emberizine-thraupid
assemblage. Within this assemblage its nearest relatives
are the richmondenines. Spiza differs from the other richmondenines
studied and resembles the emberizines and tanagers in the
possession of the muscular band which extends from the pars interna
of the m. gastrocnemius around the front of the knee. This band, in
Spiza, is smaller, however, than in any of the other species. No
icterid dissected possesses such a structure. Tordoff (1954:29)
states that Spiza is typically richmondenine in palatal structure and
makes the suggestion, with which I agree, that Spiza is a richmondenine
and may be closely related to the ancestral stock which gave
rise to the fringillid assemblage. The serological position of Spiza,
approximately equidistant from the other fringillids (Figs. 22, 23),
and the presence of the small muscular band around the front of
the knee constitute evidence supporting the central position of Spiza.
After consideration of evidence from the studies of external morphology,
ethology, myology, osteology, and serology, I propose here
an arrangement of the groups which I have studied and submit for
comparison the arrangements (of these groups) proposed by
Beecher and Tordoff. The names of subfamilies that I have been
unable to study are included in my classification and are placed in
brackets.
[Pg 207]
| Here proposed | Proposed by Tordoff (1954) on the basis of the bony palate: | Proposed by Beecher (1953) on the basis of jaw-musculature: |
| Family Ploceidae | Family Ploceidae | Family Ploceidae |
| [Subf. Bubalornithinae] | Subf. Bubalornithinae | |
| Subfamily Passerinae: distinguished from the Estrildinae by patterns of jaw-musculature (Beecher, 1953:303-304) and on the basis of comparative serology of saline-soluble proteins. | Subfamily Passerinae | Subfamily Passerinae |
| [Subfamily Ploceinae] | Subfamily Ploceinae | Subfamily Ploceinae |
| [Subfamily Viduinae] | Subfamily Viduinae | Subfamily Viduinae |
| Family Carduelidae | ||
| Subfamily Estrildinae: similar to the Carduelinae in features of the bony palate and habits (Tordoff, 1954: 18-22) and in patterns of leg-musculature and comparative serology of saline-soluble proteins. | Subfamily Estrildinae | Family Estrildidae |
| Subfamily Carduelinae: distinguished from the Fringillidae by features of the palate, geographic distribution, migration patterns, and habits (Tordoff, 1954: 14-18) and by patterns of leg-musculature and comparative serology of saline-soluble proteins. | Subfamily Carduelinae | [In Thraupidae below] |
| Family Fringillidae: all members of this family show similarities in features of the bony palate (Tordoff, 1954: 22-23), patterns of leg-musculature, and in comparative serology of saline-soluble proteins. | Family Fringillidae | Family Parulidae Subfamily Parulinae Subfamily Emberizinae |
| Family Thraupidae | ||
| Subf. Richmondeninae Subfamily Thraupinae Subfamily Emberizinae [Subfamily Fringillinae] [Subfamily Geospizinae] | Subf. Richmondeninae Subfamily Thraupinae Subfamily Fringillinae (including Emberizinae and Geospizinae) | Subfamily Pyrrhuloxiinae Subfamily Thraupinae [In Parulidae above] Subfamily Carduelinae |
[Pg 208]
It has long been recognized that the Family Fringillidae includes
some dissimilar groups. Specifically, the relationships of the subfamilies
Richmondeninae, Emberizinae, and Carduelinae of the
Family Fringillidae are poorly understood. Data from two recent
studies, one on patterns of jaw-musculature and the other on features
of the bony palate, emphasize the dissimilarity of these subfamilies
but have given rise to conflicting concepts of the relationships
of subfamilies within the Family.
This paper reports the results of studies involving morphological
and biochemical features that I consider less sensitive to external
environmental factors than are features which have been studied
previously. Patterns of leg-musculature were chosen for study because
earlier work showed that muscle patterns in the legs of passerine
birds are highly stable and vary but little. Variations, therefore,
which are consistent in separating groups of species should be
significant. Serological techniques were used because the biochemical
systems involved seem to be relatively slow to change in
response to environmental influences and because the data obtained
may be used in a highly objective manner to measure resemblance
among species.
Individual differences in the patterns of leg-musculature were
found to be slight and involved mainly the sizes and shapes of
muscles. For this reason variations involving origin, insertion, or
relative position of a muscle, were judged significant. In leg-musculature
the Richmondeninae, the Emberizinae, and the Thraupidae resemble
one another closely. Several differences in muscle pattern
were found, however, which distinguish these groups from the
Carduelinae. The leg-musculature of the carduelines closely resembles
that of the Ploceidae.
Serological techniques involved the extraction of saline-soluble
proteins from the tissues of the species to be studied. These extracts
were carefully processed and were used as antigens. Formolization
of the antigens was necessary as a means of preventing denaturation
of the proteins by enzymatic activity. Antisera were produced in
rabbits. The method of testing involved turbidimetric analysis of
the precipitin reaction. Utilizing the values for the precipitin tests
a model was constructed which showed the relationships of the
eleven species used in these tests. From a study of the model and
the data used in its construction, it was determined that the Richmondeninae,
Emberizinae, and Thraupidae constitute an assemblage
[Pg 209]
distinct from the other species studied. The Carduelinae are
excluded from the assemblage and serologically are most closely related
to the Estrildinae. The estrildines, serologically, do not closely
resemble Passer, Subfamily Passerinae, although recent classifications
place these two subfamilies in the Family Ploceidae.
Upon consideration of all evidence now available—from external
morphology, ethology, myology, osteology, and serology—several
hypotheses regarding the relationships of the groups studied are set
forth. The richmondenines, emberizines, and tanagers are closely
related subfamilies and are here included in the Family Fringillidae.
The Estrildinae and Carduelinae are closely related subfamilies, but
neither group is closely related to the Passerinae. The estrildines
and carduelines, therefore, are placed in a separate family, the
Carduelidae. In some ways, Spiza is an aberrant member of the Subfamily
Richmondeninae but should be retained in that subfamily.
It is suggested that Spiza is a primitive richmondenine closely related
to the ancestral fringillid stock.
[Pg 210]
[Pg 211]
Transmitted June 8, 1954.
25-4632
[Pg i]
MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this
series by addressing the Exchange Librarian, University of Kansas Library,
Lawrence, Kansas. Copies for individuals, persons working in a particular
field of study, may be obtained by addressing instead the Museum of Natural
History, University of Kansas, Lawrence, Kansas. There is no provision for
sale of this series by the University Library, which meets institutional requests,
or by the Museum of Natural History, which meets the requests of individuals.
Nevertheless, when individuals request copies from the Museum, 25 cents should
be included, for each separate number that is 100 pages or more in length, for
the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum's supply (not the Library's
supply) is exhausted. Numbers published to date, in this series, are as follows:
| Vol. 1. | 1. | The pocket gophers (Genus Thomomys) of Utah. By Stephen D. Durrant. Pp. 1-82, 1 figure in text; August 15, 1946. |
| 2. | The systematic status of Eumeces pluvialis Cope, and noteworthy records of other amphibians and reptiles from Kansas and Oklahoma. By Hobart M. Smith. Pp. 85-89. August 15, 1946. | |
| 3. | The tadpoles of Bufo cognatus Say. By Hobart M. Smith. Pp. 93-96, 1 figure in text. August 15, 1946. | |
| 4. | Hybridization between two species of garter snakes. By Hobart M. Smith. Pp. 97-100. August 15, 1946. | |
| 5. | Selected records of reptiles and amphibians from Kansas. By John Breukelman and Hobart M. Smith. Pp. 101-112. August 15, 1946. | |
| 6. | Kyphosis and other variations in soft-shelled turtles. By Hobart M. Smith. Pp. 117-124, 3 figures in text. July 7, 1947. | |
| *7. | Natural history of the prairie vole (Mammalian Genus Microtus). By E. W. Jameson, Jr. Pp. 125-151, 4 figures in text. October 6, 1947. | |
| 8. | The postnatal development of two broods of great horned owls (Bubo virginianus). By Donald F. Hoffmeister and Henry W. Setzer. Pp. 157-173, 5 figures in text. October 6, 1947. | |
| 9. | Additions to the list of the birds of Louisiana. By George H. Lowery, Jr. Pp. 177-192. November 7, 1947. | |
| 10. | A check-list of the birds of Idaho. By M. Dale Arvey. Pp. 193-216. November 29, 1947. | |
| 11. | Subspeciation in pocket gophers of Kansas. By Bernardo Villa R. and E. Raymond Hall. Pp. 217-236, 2 figures in text. November 29, 1947. | |
| 12. | A new bat (Genus Myotis) from Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 237-244, 6 figures in text. December 10, 1947. | |
| 13. | Tadarida femorosacca (Merriam) in Tamaulipas, Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 245-248, 1 figure in text. December 10, 1947. | |
| 14. | A new pocket gopher (Thomomys) and a new spiny pocket mouse (Liomys) from Michoacán, México. By E. Raymond Hall and Bernardo Villa R. Pp. 249-256, 6 figures in text. July 26, 1948. | |
| 15. | A new hylid frog from eastern Mexico. By Edward H. Taylor. Pp. 257-264, 1 figure in text. August 16, 1948. | |
| 16. | A new extinct emydid turtle from the Lower Pliocene of Oklahoma. By Edwin C. Galbreath. Pp. 265-280, 1 plate. August 16, 1948. | |
| 17. | Pliocene and Pleistocene records of fossil turtles from western Kansas and Oklahoma. By Edwin C. Galbreath. Pp. 281-284. August 16, 1948. | |
| 18. | A new species of heteromyid rodent from the Middle Oligocene of northeastern Colorado with remarks on the skull. By Edwin C. Galbreath. Pp. 285-300, 2 plates. August 16, 1948. | |
| 19. | Speciation in the Brazilian spiny rats (Genus Proechimys, Family Echimyidae). By João Moojen. Pp. 301-406, 140 figures in text. December 10, 1948. | |
| 20. | Three new beavers from Utah. By Stephen D. Durrant and Harold S. Crane. Pp. 407-417, 7 figures in text. December 24, 1948. | |
| 21. | Two new meadow mice from Michoacán, Mexico. By E. Raymond Hall. Pp. 423-427, 6 figures in text. December 24, 1948. | |
| 22. | An annotated check list of the mammals of Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa R. Pp. 431-472, 2 plates, 1 figure in text. December 27, 1949. | |
| 23. | Subspeciation in the kangaroo rat, Dipodomys ordii. By Henry W. Setzer. Pp. 473-573, 27 figures in text, 7 tables. December 27, 1949. | |
| 24. | Geographic range of the hooded skunk, Mephitis macroura, with description of a new subspecies from Mexico. By E. Raymond Hall and Walter W. Dalquest. Pp. 575-580, 1 figure in text. January 20, 1950. | |
| [Pg ii] | 25. | Pipistrellus cinnamomeus Miller 1902 referred to the Genus Myotis. By E. Raymond Hall and Walter W. Dalquest. Pp. 581-590, 5 figures in text. January 20, 1950. |
| 26. | A synopsis of the American bats of the Genus Pipistrellus. By E. Raymond Hall and Walter W. Dalquest. Pp. 591-602, 1 figure in text. January 20, 1950. | |
| Index. Pp. 605-638. | ||
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
| 4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | 1. | Preliminary survey of a Paleocene faunule from the Angels Peak area, New Mexico. By Robert W. Wilson. Pp. 1-11, 1 figure in text. February 24, 1951. |
| 2. | Two new moles (Genus Scalopus) from Mexico and Texas. By Rollin H. Baker. Pp. 17-24. February 28, 1951. | |
| 3. | Two new pocket gophers from Wyoming and Colorado. By E. Raymond Hall and H. Gordon Montague. Pp. 25-32. February 28, 1951. | |
| 4. | Mammals obtained by Dr. Curt von Wedel from the barrier beach of Tamaulipas, Mexico. By E. Raymond Hall. Pp. 33-47, 1 figure in text. October 1, 1951. | |
| 5. | Comments on the taxonomy and geographic distribution of some North American rabbits. By E. Raymond Hall and Keith R. Kelson. Pp. 49-58. October 1, 1951. | |
| 6. | Two new subspecies of Thomomys bottae from New Mexico and Colorado. By Keith R. Kelson. Pp. 59-71, 1 figure in text. October 1, 1951. | |
| 7. | A new subspecies of Microtus montanus from Montana and comments on Microtus canicaudus Miller. By E. Raymond Hall and Keith R. Kelson. Pp. 73-79. October 1, 1951. | |
| 8. | A new pocket gopher (Genus Thomomys) from eastern Colorado. By E. Raymond Hall. Pp. 81-85. October 1, 1951. | |
| 9. | Mammals taken along the Alaskan Highway. By Rollin H. Baker. Pp. 87-117, 1 figure in text. November 28, 1951. | |
| *10. | A synopsis of the North American Lagomorpha. By E. Raymond Hall. Pp. 119-202, 68 figures in text. December 15, 1951. | |
| 11. | A new pocket mouse (Genus Perognathus) from Kansas. By E. Lendell Cockrum. Pp. 203-206. December 15, 1951. | |
| 12. | Mammals from Tamaulipas, Mexico. By Rollin H. Baker. Pp. 207-218. December 15, 1951. | |
| 13. | A new pocket gopher (Genus Thomomys) from Wyoming and Colorado. By E. Raymond Hall. Pp. 219-222. December 15, 1951. | |
| 14. | A new name for the Mexican red bat. By E. Raymond Hall. Pp. 223-226. December 15, 1951. | |
| 15. | Taxonomic notes on Mexican bats of the Genus Rhogeëssa. By E. Raymond Hall. Pp. 227-232. April 10, 1952. | |
| 16. | Comments on the taxonomy and geographic distribution of some North American woodrats (Genus Neotoma). By Keith R. Kelson. Pp. 233-242. April 10, 1952. | |
| 17. | The subspecies of the Mexican red-bellied squirrel, Sciurus aureogaster. By Keith R. Kelson. Pp. 243-250, 1 figure in text. April 10, 1952. | |
| 18. | Geographic range of Peromyscus melanophrys, with description of new subspecies. By Rollin H. Baker. Pp. 251-258, 1 figure in text. May 10, 1952. | |
| 19. | A new chipmunk (Genus Eutamias) from the Black Hills. By John A. White. Pp. 259-262. April 10, 1952. | |
| 20. | A new piñon mouse (Peromyscus truei) from Durango, Mexico. By Robert B. Finley, Jr. Pp. 263-267. May 23, 1952. | |
| 21. | An annotated checklist of Nebraskan bats. By Olin L. Webb and J. Knox Jones, Jr. Pp. 269-279. May 31, 1952. | |
| 22. | Geographic variation in red-backed mice (Genus Clethrionomys) of the southern Rocky Mountain region. By E. Lendell Cockrum and Kenneth L. Fitch. Pp. 281-292, 1 figure in text. November 15, 1952. | |
| 23. | Comments on the taxonomy and geographic distribution of North American microtines. By E. Raymond Hall and E. Lendell Cockrum. Pp. 293-312. November 17, 1952. | |
| [Pg iii] | 24. | The subspecific status of two Central American sloths. By E. Raymond Hall and Keith R. Kelson. Pp. 313-337. November 21, 1952. |
| 25. | Comments on the taxonomy and geographic distribution of some North American marsupials, insectivores, and carnivores. By E. Raymond Hall and Keith R. Kelson. Pp. 319-341. December 5, 1952. | |
| 26. | Comments on the taxonomy and geographic distribution of some North American rodents. By E. Raymond Hall and Keith R. Kelson. Pp. 343-371. December 15, 1952. | |
| 27. | A synopsis of the North American microtine rodents. By E. Raymond Hall and E. Lendell Cockrum. Pp. 373-498, 149 figures in text. January 15, 1953. | |
| 28. | The pocket gophers (Genus Thomomys) of Coahuila, Mexico. By Rollin H. Baker. Pp. 499-514, 1 figure in text. June 1, 1953. | |
| 29. | Geographic distribution of the pocket mouse, Perognathus fasciatus. By J. Knox Jones, Jr. Pp. 515-526, 7 figures in text. August 1, 1953. | |
| 30. | A new subspecies of wood rat (Neotoma mexicana) from Colorado. By Robert B. Finley, Jr. Pp. 527-534, 2 figures in text. August 15, 1953. | |
| 31. | Four new pocket gophers of the genus Cratogeomys from Jalisco, Mexico. By Robert J. Russell. Pp. 535-542. October 15, 1953. | |
| 32. | Genera and subgenera of chipmunks. By John A. White. Pp. 543-561, 12 figures in text. December 1, 1953. | |
| 33. | Taxonomy of the chipmunks, Eutamias quadrivittatus and Eutamias umbrinus. By John A. White. Pp. 563-582, 6 figures in text. December 1, 1953. | |
| 34. | Geographic distribution and taxonomy of the chipmunks of Wyoming. By John A. White. Pp. 584-610, 3 figures in text. December 1, 1953. | |
| 35. | The baculum of the chipmunks of western North America. By John A. White. Pp. 611-631, 19 figures in text. December 1, 1953. | |
| 36. | Pleistocene Soricidae from San Josecito Cave, Nuevo Leon, Mexico. By James S. Findley. Pp. 633-639. December 1, 1953. | |
| 37. | Seventeen species of bats recorded from Barro Colorado Island, Panama Canal Zone. By E. Raymond Hall and William B. Jackson. Pp. 641-646. December 1, 1953. | |
| Index. Pp. 647-676. | ||
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | *1. | Mammals of Kansas. By E. Lendell Cockrum. Pp. 1-303, 73 figures in text, 37 tables. August 25, 1952. |
| 2. | Ecology of the opossum on a natural area in northeastern Kansas. By Henry S. Fitch and Lewis L. Sandidge. Pp. 305-338, 5 figures in text. August 24, 1953. | |
| 3. | The silky pocket mice (Perognathus flavus) of Mexico. By Rollin H. Baker. Pp. 339-347, 1 figure in text. February 15, 1954. | |
| 4. | North American jumping mice (Genus Zapus). By Philip H. Krutzsch. Pp. 349-472, 47 figures in text, 4 tables. April 21, 1954. | |
| 5. | Mammals from Southeastern Alaska. By Rollin H. Baker and James S. Findley. Pp. 473-477. April 21, 1954. | |
| 6. | Distribution of Some Nebraskan Mammals. By J. Knox Jones, Jr. Pp. 479-487. April 21, 1954. | |
| 7. | Subspeciation in the montane meadow mouse, Microtus montanus, in Wyoming and Colorado. By Sydney Anderson. Pp. 489-506, 2 figures in text. July 23, 1954. | |
| 8. | A new subspecies of bat (Myotis velifer) from southeastern California and Arizona. By Terry A. Vaughn. Pp. 507-512. July 23, 1954. | |
| 9. | Mammals of the San Gabriel mountains of California. By Terry A. Vaughn. Pp. 513-582, 1 figure in text, 12 tables. November 15, 1954. | |
| More numbers will appear in volume 7. | ||
| Vol. 8. | 1. | Life history and ecology of the five-lined skink, Eumeces fasciatus. By Henry S. Fitch. Pp. 1-156, 26 figs. in text. September 1, 1954. |
| 2. | Myology and serology of the Avian Family Fringillidae, a taxonomic study. By William B. Stallcup. Pp. 157-211, 23 figures in text, 4 tables. November 15, 1954. | |
| More numbers will appear in volume 8. | ||
Featured Books

Chivalry: Dizain des Reines
James Branch Cabell
setting his expertness in genealogyto the genial task of devising a family tree for his figures offi...

News from Nowhere; Or, An Epoch of Rest
William Morris
eachothers’ opinions (which could scarcely be expected ofthem), at all events did not always attem...

Descriptions of New Hylid Frogs From Mexico and Central America
William Edward Duellman
science for many years, andmost of the novelties today are found in the less accessible highlands.N...

The Amphibians and Reptiles of Michoacán, México
William Edward Duellman
mphibians in México. Some partsof the country, because of their accessibility, soon became relative...

Mammals of Mesa Verde National Park, Colorado
Sydney Anderson
e canyons are roughly parallel and all open into thecanyon of the Mancos River, which forms the sout...

Samantha among the Brethren — Volume 4
Marietta Holley
stands to reason it is. And I'd like to knowwhat you have got to say about him any way?"Sez I, "That...

A Bayard From Bengal
F. Anstey
he Unwieldy Gifthorse48VIII.A Rightabout Facer for Mr Bhosh55IX.The Dark Horse63X.Trust Her Not! She...

Three Men and a Maid
P. G. Wodehouse
lid black; /* a thin black line border.. */ padding: 6px; /* ..spaced a bit out from the gr...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Myology and Serology of the Avian Family Fringillidae: A Taxonomic Study" :